Tripropylene Glycol Methyl Ether, usually shortened to TPM or TPGME, did not just drop into industrial laps by accident. Chemists in the mid-20th century, always reaching for better, cleaner solvents for coatings and cleaning, pushed for alternatives with better solvency and manageable evaporation rates. Between the messy volatility of single glycol ethers and the environmental headaches of older solvents, researchers zeroed in on ethers like TPM. Propylene oxide oligomers, already under the microscope for their flexibility, formed the backbone. Early manufacturers, mainly in the US and Europe, ramped up TPM production as paints, inks, and cleaning products moved away from high-toxicity options. As demand grew, so did global shipments. Today, facilities in Asia routinely churn out tons, driven by both economic and regulatory motives.
TPM walks a line most chemicals never reach. This clear, practically odorless liquid flows easily, making life simple for anyone pouring 200-liter drums in a plant or blending it into a water-based paint. It straddles both polar and non-polar worlds—dissolving grease just as smoothly as it lifts dye. The product found its feet in the 1980s as an upgrade over mono- and di-propylene glycol ethers, balancing drying time, worker exposure, and final finish. Laboratories order the technical grade for paint stripping, gravitating toward TPM’s low volatility. Paint manufacturers, textile finishers, and ink blenders all keep it on speed dial. Over time, TPM gained a strong following not thanks to aggressive marketing, but because it gets the job done without much drama or extra handling.
TPM weighs in at a molecular mass close to 190–210 g/mol, depending on its isomer mix. At room temperature it stays perfectly clear, which factory workers and lab managers both appreciate. Its boiling point—a steady 245-250°C—puts it out of range of your average flammable solvent, cutting fire risks in crowded workspaces. TPM’s vapor pressure hovers low, limiting inhalation risk in non-ventilated rooms. With a flash point above 110°C, the risk of accidental ignition drops. Its solubility, blending right into water in any ratio, gives it an edge over cheaper ethers with awkward phase splits. Chemical resistance stays strong across a variety of acids and mild bases, so it sticks around even in harsh, caustic environments. The low odor is not just for convenience. It reduces the sensory overload common in older solvent plants and makes TPM attractive for consumer-facing products.
Manufacturers ship TPM under standard UN codes for glycol ethers, with most suppliers labeling the product by CAS number 25498-49-1. Safety Data Sheets flag its status as a moderately hazardous material, reminding users to glove up and avoid direct splashes. TPM comes in purity grades: technical runs above 98%, and high-purity versions for semiconductor or specialty coatings edge higher. Color ratings matter, so spec sheets always include an APHA or Pt/Co number, usually below 20. Water content, measured by Karl Fischer titration, rarely exceeds 0.1% in good stock. Drums or IBCs sport labels listing batch numbers, dates, and both GHS hazard and precaution pictograms—because regulators, not to mention customers, refuse to gamble with hidden compositions or sloppy transport.
Production starts on an industrial scale with propylene oxide oligomerization—think chemistry with purpose, not guesswork. Manufacturers push propylene oxide through catalysis to create tripropylene glycol, all while controlling temperature and pressure to keep chains at the right length. Once the glycol fraction sits ready, methylation steps in. Methanol reacts under slightly basic or acidic conditions, often in a reactor lined with stainless steel. The final mixing, distillation, and polishing steps remove water and residual methanol, leaving TPM clear and pure. Many producers re-circulate unused starting materials, so the process fits green chemistry ideals better than older solvent lines that dumped streams of waste. This efficiency means thick catalogs of quality-control results at each step, with final product bottles checked for purity and moisture before getting packed for shipment.
In the workplace, TPM holds its structure well. Direct strong acids or aggressive oxidants start to break it down, generating traces of formaldehyde and other low-molecular aldehydes. Under mild conditions, TPM resists hydrolysis. Formulators do not view it as a reactive intermediate, but rather as a stable solvent that can serve as a carrier for resins or dyes. Blending TPM with other glycol ethers or amines creates ready-to-use paint thinners or metal-cleaning cocktails. Researchers exploring new generations of coatings experiment with TPM derivatives, swapping out the methyl group for longer chains, trying to tweak evaporation rate or compatibility. TPM can form esters under forceful reaction conditions, but most applications just take it as-is, leaning on consistency and predictability.
The chemical might show up in paperwork as TPGME, TPM, or tripropylene glycol mono methyl ether. Some suppliers stick to the IUPAC name—2-methoxy-1-(2-methoxypropoxy)propane—although in most industry catalogs, brevity wins. International shipments sometimes list local trade names, like Dow’s “TPM Solvent” or BASF’s technical solvents range, without much deviation between regions. Tracking synonyms can feel tedious, but for logistics and labeling managers, missing a name spells compliance headaches. Import paperwork in China or India occasionally throws in further abbreviations, so sharp-eyed warehouse staff check every drum before blending or repacking.
Every TPM drum carries the standard hazard warnings—eye protection and gloves always up front because glycol ethers can slip through bare skin. Factory managers run local exhaust fans in the blending areas. TPM’s low volatility makes spills less dramatic than, say, acetone, but any glycol ether carries some risk of absorption and overexposure. Emergency procedures call for quick containment, with spill kits standing by. Both OSHA and the EU list TPM on substance registries, so safety audits include vapor monitoring and skin checks for workers. Regular training on proper decanting and shower facilities nearby are standard. TPM storage stays stable in regular steel drums, but moisture must stay low to avoid contamination. Large-scale plants often adopt closed systems, putting distance between workers and solvents without losing hands-on efficiency.
Real value shows in the broad spread of TPM’s day jobs. Paint and coating factories rely on it to improve flow and cut brush marks, turning awkward surfaces into smooth, easy-to-wipe finishes. In the cleaning sector, TPM outperforms cheaper glycols by stripping tough industrial grime but leaving less sticky residue. Printers and ink makers hold onto TPM for its control over drying time—just enough to let technicians run detailed graphics without smearing, not so fast as to gum up equipment. Textile dyeing operations like the solvent’s stable pH, keeping bright colors from fading or washing out. Pharmaceutical labs sometimes reach for TPM as a carrier for actives during formulation trials. It doesn’t fit every niche, but nearly every major commodity sector keeps some on hand, from electronics through adhesives and automotive detailing. Its friendly blend of solvency, low odor, and moderate evaporation earns repeat business.
Lab benches buzz with ideas for tweaking TPM’s core structure. Scientists keep pushing to make glycol ethers safer for long-term use. Some test adding UV stabilizers to reduce photo-degradation, stretching shelf life for sensitive coatings. Others search for ways to lower TPM’s toxicity profile, dialing down exposure risk to keep regulatory agencies satisfied. Green chemistry drives plenty of university labs to look for bio-based propylene oxide, allowing future TPM runs to use corn or waste biomass rather than fossil feedstocks. The struggle lies in balancing performance with demands for cleaner environmental impact; nobody wants trade-offs that bring back old fire and toxicity hazards. Recent advances in catalyst design hint at more efficient reaction processes—cutting energy and raw material use by impressive margins. All these efforts feed into one goal: delivering safer, better-performing solvents without costing the Earth in dollars or emissions.
Toxicologists, rightly cautious around glycol ethers, scrutinize every batch for impurities and long-term exposure effects. TPM absorbs through the skin but much less aggressively than shorter-chain cousins like ethylene glycol ethers. Short-term inhalation or handling rarely causes immediate problems, beyond mild eye redness or skin dryness. Chronic exposure data tells a mixed story—animal studies show some liver and kidney strain after sustained heavy doses, but regulatory exposure limits sit comfortably well below dangerous thresholds. TPM rates better than many historical solvents, partly from its higher molecular weight and slower uptake in body tissues. Ongoing studies explore next-generation blood markers for glycol-ether exposure, picking up even tiny traces in factory workers. Regulatory agencies update guidelines regularly, checking data from worker health screenings and chemical fate studies in the environment. TPM still outpaces older competitors for both acute and chronic standards, but regulators and companies alike know not to rest easy.
The direction for TPM doesn’t sit behind closed doors. Regulatory pressure keeps steering development toward safer, greener chemical options. More companies ask about plant-based propylene oxide for the glycol backbone, looking to reduce dependence on fossil fossil feedstocks and shrink their greenhouse gas footprints. Paint and cleaning supply firms expect solvents that perform as well as TPM without raising red flags for worker health. At least a handful of companies now trial closed-loop solvent recovery, re-using and purifying TPM after each use to cut waste streams and supply costs. Automation edges in, limiting direct contact and supporting worker protection. Meanwhile, research into structurally similar compounds pushes for an ever-better balance of evaporation rate, solvency, and hazard. TPM’s track record for reliability and versatility sets a high bar, but no one buying chemicals in bulk, managing compliance, or tracking workplace health lets their guard down. The market asks for more than a one-size-fits-all answer and TPM, for now, keeps rising to those requests, ready to evolve as fast as science and regulation demand.
Tripropylene glycol methyl ether, or TPM for short, slips into all kinds of products we use every day. Most folks don’t know the name, but they run into it at the hardware store, in fresh paint, or on glossy furniture. Walk past freshly coated walls and you’re probably catching a whiff of it. This chemical carries a long name, but it falls into a big group: glycol ethers. TPM acts as a strong yet manageable solvent, floating easily through liquids and mixing into formulas where others clump or fail.
Paint makers rely on TPM because it can loosen pigments without making everything as thin as water. Try painting in the middle of summer, and TPM helps slow the drying just enough to keep the finish streak-free. Office workers who recently upgraded desks might find it hidden in the coatings that stop fingerprints from sticking all day. Glass cleaners also often include TPM, making sure the spray slides away fingerprints and leaves glass invisible again. Even auto cleaners and degreasers soak up a fair share. TPM stands out not just for what it dissolves, but for what it leaves behind—little smell, and little stickiness.
Lots of jobs in industry involve getting oil and grease to release their grip. TPM proves its worth here. I’ve watched janitors scrub a kitchen where layers of grease had taken over every knob. The right degreaser made with TPM turned a tough morning into an easy one. Factories often use it for their own tough spills. They like that TPM doesn’t just cut through grime; it also evaporates at a moderate pace, so workers aren’t stuck waiting around for a surface to dry or risking fire from fumes.
I’ve seen workers in printing shops dipping cloths into inks and cleaners, trusting TPM to clear excess pigment without breaking down the tools they clean. Ink technology benefits because TPM holds on to pigment and resin, making it easier to get a sharp print on paper and packaging. The balance here matters—too harsh, and you melt the machinery; too weak, and you’re scrubbing all day.
Though the chemical helps a lot of industries, it still carries some risks. Breathing in lots of vapor on a hot day, or splashing it on bare skin, invites trouble. Some people react with redness or spots. Long hours in a poorly ventilated shop can lead to headaches. Workers need reliable gloves, open windows, or air systems. TPM might not be the most hazardous chemical in a toolbox, but safety rules still matter, as sore skin or lightheadedness aren’t worth ignoring. Some regulations tell factories how much TPM they should let float in the air; more places should test and follow up.
TPM wins over many because it can substitute for older, harsher chemicals. Solvents from years back were rough on people and the planet. TPM, used wisely, rarely lingers in the environment, but disposal still calls for attention. Industrial sites need sturdy rules on how they handle leftovers. Cities and states can help by checking that disposal centers really keep solvents out of the water supply.
Shops and factories can fit new fans and better filters to suck away vapor. Not every place welcomes expensive overhauls, but investment in health beats dealing with accidents and slow-downs. Product makers can keep searching for greener solvents. Each new blend, with lower evaporation and milder fumes, matters to workers and families. People choosing paints, cleaners, or coatings have a say too—reading labels and picking brands that take worker safety and clean air seriously.
Tripropylene Glycol Methyl Ether sounds like something cooked up in a lab for the sole purpose of making regular folks uneasy. Truth is, it’s no backyard chemical, and you might find it in more places than you’d expect. From household cleaners, paints, and coatings, down to some personal care products, it shows up in different corners of daily life. Folks have the right to ask: Is it actually safe?
Most manufacturers like this solvent because it dissolves grease and dries without leaving a harsh smell behind. That’s handy in paints and surface cleaners. Working in auto shops, I’ve opened plenty of cans with this stuff inside. Never saw much fuss during application—nobody passed out, nobody’s hands started shedding skin. Still, glove use matters. No solvent feels “gentle” for long with constant contact.
The American Conference of Governmental Industrial Hygienists (ACGIH) set an exposure limit for this chemical. That step says something. Limitations aren’t just red tape—they’re lifelines. Too much vapor, too often, can cause dizziness, headaches, or skin and eye irritation. Getting splashed or sitting in fumes for hours isn’t part of most people’s day, though. In an office or at home, you’d need a major spill or foolish misuse for real trouble. In workplaces, I always saw safety data sheets and extraction fans keeping risks down.
Research has scratched at both long-term and short-term effects. The biggest issues seem to come when people breathe large amounts in closed spaces, or dunk their skin in the liquid too much. Animal studies showed some effect on liver and kidneys at high doses, but those doses run higher than any regular public would hit by mopping a floor or painting a wall. Still, sensitive groups—kids, folks with asthma, or people who get rashes easy—might feel more irritation, so some caution makes sense.
The good news: Most health problems linked with this solvent don’t happen to careful users. Reading product labels matters, always keeping spaces aired out matters too. Gloves and goggles aren’t just for show in shops where this chemical gets heavy use. At home, I’ve seen people go through a spring-cleaning frenzied with every window closed tight. That’s asking for a headache, no matter what cleaner you use. Common sense fixes most problems—open a window, wear gloves, avoid sniffing containers.
Countries like the US and those in Europe require hazard labeling. So, any product with a meaningful amount of this ether tells users it isn’t water—treat it with respect. A little planning goes a long way. Don’t store it within easy reach of kids or pets, and keep containers sealed. Folks with specific medical conditions should ask a doctor if repeated use causes problems.
Plenty of greener products have hit the shelves. Some swap this glycol ether for citrus-based solvents or plain soap and water where they work well. That gives options to those who prefer to dodge synthetic chemical names. But on tough grease and industrial messes, old-fashioned elbow grease or homemade cleaners sometimes just can’t cut it, and that’s where these tools keep their edge. The key comes down to using the right tool for the job, following instructions, and treating these chemicals with the healthy caution they deserve.
Tripropylene Glycol Methyl Ether often turns up behind the scenes in everything from paints to cleaners and printing inks. Its popularity grows from its reliability, but that same convenience hides a few important points about keeping it safe. Over the years, I’ve seen more than one warehouse manager underestimate chemical risks and end up in sticky situations—sometimes literally, with leaks or fumes. So it’s time to focus on what really matters about safe storage and handling.
Ignoring proper storage can spell trouble fast. A container left open or stored under the wrong conditions can release fumes, with headaches and dizziness showing up for workers before anyone realizes there’s a problem. Stores without good airflow or temperature control increase the chance of fire because Tripropylene Glycol Methyl Ether is combustible. The flash point sits around 93°C (200°F). For safety, I always encourage storing it away from sparks or direct sunlight, and definitely far from heat sources.
Containers should be tightly sealed. I remember once seeing a barrel left half-open in a back room—the place stank, and worse, the liquid inside started to turn cloudy. Contamination like this doesn’t just lower quality, it also raises the odds of unpredictable reactions or corrosion.
A smart approach is simple: Keep containers in a dry, shady spot with steady temperatures—between 2°C and 40°C works well. Humidity lets condensation build up, which can accelerate container rust or corrosion. Steel drums or high-density polyethylene containers with clear hazard labeling usually hold up the best. Shelving and racks should support all weights without wobbling. Leaks left unnoticed might soak through cardboard or thin plastics, so sturdy materials become non-negotiable.
Fire codes don’t just exist for paperwork. This chemical belongs in storage away from oxidizers and acids since reactions with these can get dangerous. Equip every storage site with a tested fire extinguisher nearby—carbon dioxide or dry powder units handle chemical fires best.
Some workers make the mistake of loading heavy drums near an emergency exit. Cluttering exits blocks escape during an emergency, and cramped spaces also make handling tools awkward. Plan storage with wide walkways and keep exits fully open.
Direct skin contact or breathing vapor isn’t just uncomfortable—it’s dangerous over time. I’ve seen rough, red hands from shortcuts and headaches from skipping proper ventilation. Use gloves made from nitrile or butyl rubber and always wear splash goggles since accidents with splashing or spills happen more often than most expect.
Working indoors without good exhaust systems risks vapor buildup. Simple local fume hoods or mechanical ventilation make a big difference. Safety showers and eye wash stations near work areas help in case of accidental splashes. Even a few minutes’ delay can lead to lasting injuries.
Small drips can add up to slippery floors and contaminated surfaces. Absorb spills with sand or commercial pads. Shove paper towels aside for housekeeping—proper clean-up matters. Waste containers with tight lids cut down on vapor release, and pairing chemical waste with local hazardous waste services prevents runoff into sewers or drains. A casual toss into the regular trash creates real headache for sanitation crews and can foul up local water systems.
Safety talks and visible reminders give everyone a stake in chemical care. Sometimes, the difference comes down to reminding new hires of the rules without making them feel embarrassed. Assigning a go-to person for chemical storage in each shift also helps—just as team sports need a captain, the workplace does better with clear point people on safety.
Treating Tripropylene Glycol Methyl Ether with respect means no surprises for workers, no chemical fires, and no sick days from careless mistakes. With the right balance of caution, organization, and teamwork, it becomes just another manageable, useful tool on the shop floor.
People working in labs or running cleaning businesses often ask about mixing different liquids. Tripropylene Glycol Methyl Ether, also known as TPM, pops up a lot in paints, coatings, and cleaning solutions. Curiosity about how this chemical interacts with water isn't just trivia. It has a direct impact on shelf life, safety, and cleaning power.
Let’s start with the practical question: does TPM actually mix with water? Yes, it does. There’s no need for fancy mixing tanks or warming the chemical to get a single-phase solution. TPM molecules share enough in common with water—their "polarity"—to blend together smoothly. As someone who’s done plenty of cleanup work with chemical solvents, this property takes a lot off my mind. Instead of worrying about separation and streaks, I can count on an even job.
Anybody dealing with stubborn grime, painting projects, or industrial formulas cares about how their solvents behave. I've cleaned everything from office carpets to greasy auto parts, and the wrong chemical combo can either leave a foggy mess or do nothing at all. When you mix TPM with water, you get consistent performance. Cleaning staff can dilute TPM-containing detergents without fretting over losing punch. Folks in paint shops can add water to TPM blends for better flow or easier equipment wash-up.
Mixing two liquids doesn’t always go this well. Take oil and vinegar—they separate fast and need constant shaking for any hope of blending. Not the case with TPM and water. This is more like sugar in your coffee—stir once and it stays mixed. That reliability lets product makers cut down on stabilizers and additives, sometimes reducing costs and waste.
The story doesn’t end with performance or ease of mixing. TPM’s miscibility makes it easier to flush equipment, tanks, and even hands when spills happen. You can rinse most residues down with water, cutting down the risk of fire or toxic buildup. That’s huge in busy labs or maintenance shops, where accidents cost time and money.
That said, don’t let miscibility trick you into being careless. Even if TPM blends easily with water, dumping it down the drain is bad news for rivers and fish. Some cities already monitor solvent discharge closely, and with good reason. Users ought to look for proper waste collection or treatment, not just a quick water rinse.
I’ve seen some progress over the years toward using less harsh chemicals with similar blending properties. Bio-based solvents are stepping in, sometimes replacing TPM entirely, but they're not a universal fix yet. Some jobs still demand what TPM can do—cleaning, flow control, or blending. Maybe the best route now is using TPM only where its water miscibility genuinely pays off, and training workers to handle it responsibly. Technologies for solvent recovery and recycling are getting better, and wider adoption in small shops would help.
For now, if the task involves dissolving, thinning, or cleaning, knowing that TPM and water work together without fuss can make life a lot easier—and maybe a bit safer, too.
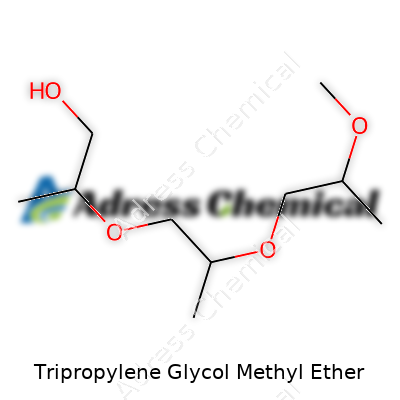
Walk through a paint factory or a printing plant, and odds are you’ll trip over barrels labeled "TPM." Behind those three letters sits Tripropylene Glycol Methyl Ether. It wears a pretty technical name, but beneath that, it works as a common solvent. Guys like me, poking around in workshop corners, like to know what they’re handling. Tripropylene Glycol Methyl Ether runs under the CAS number 25498-49-1 and its chemical formula is C10H22O4.
CAS numbers show up in every corner of chemical supply chains. They’re like handshakes—firm, unique, and hard to fake. Try searching “Tripropylene Glycol Methyl Ether” across different brands, and you might get different names. There’s PnB, TPM, or Propasol. But that CAS number—25498-49-1—ties everything back together, cutting through the marketing fluff. That matters for anyone, from lab managers to environmental inspectors, looking to avoid confusion or dangerous mix-ups.
You can’t really avoid TPM if you’re in coatings, inks, or cleaners. This stuff often shows up in water-based paints, thanks to its knack for dissolving both oil and water-loving stuff. Personal experience working with DIY cleaning products showed me that TPM never seems to complain—the solution just turns out smooth and streak-free. Print shops, too, lean on TPM. Printers run long hours, and the presses spit out signature after signature. They rely on solvents that don’t quit or ruin the job halfway through.
Like a lot of solvents, TPM needs careful handling. I've seen folks pop open drums without gloves or masks, which turns risky fast. Getting TMP on your skin might not raise alarms at first, but do it enough, and the irritation piles up. The vapor shouldn’t get a free pass into your lungs either. Working in a tightly closed-off space, breathing in that faintly sweet smell, headaches sneak up on you. Safety data sheets, like the one for this chemical, spell out the risks: always ventilate well, suit up with gloves, and avoid careless spills.
Waste disposal jumped to mind the first time our workshop had to clear out old solvents. Used to be, folks poured stuff down the drain without a second thought. Now, TPM’s place in water-based cleaning products pushes people to rethink what happens after dumping—the effects stack up in streams and lakes nearby. Local rules around hazardous waste keep tightening, forcing shops and households alike to send leftover slops to specialized collection sites.
The real trick with chemicals like TPM lies in striking a balance. You want their cleaning power and easy mixing—but not at any cost. I’ve watched product designers hunt for alternatives that clean just as well, yet ease stress on workers and waterways. Some green chemistry projects start swapping out solvents altogether, trialing plant-based ones. Costs can bump up at the start, and the smell sometimes throws people for a loop. Over the long haul, though, those shifts can build safer workplaces and help out the wider community.
At the end of the day, knowing a CAS number like 25498-49-1 and a chemical sketch like C10H22O4 doesn’t just arm someone with trivia. Understanding the chemistry, weighing safety precautions, keen disposal habits, and keeping an eye on safer substitutes turns a chemical from a risk into a partner. We can treat TPM with respect while keeping eyes open for ways to limit its downsides, inside factories and out in the world.
| Names | |
| Preferred IUPAC name | 1-methoxy-2-(2-methoxypropoxy)propane |
| Other names |
TPM TPGME 1-Methoxy-2-(2-(2-methoxypropoxy)propoxy)propane Methoxytripropylene glycol 3-(2-(2-Methoxypropoxy)propoxy)-1-methoxypropane |
| Pronunciation | /traɪ-proʊˈpiː-liːn ˈɡlaɪ-kɒl ˈmɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 25498-49-1 |
| Beilstein Reference | 1089926 |
| ChEBI | CHEBI:82257 |
| ChEMBL | CHEMBL38330 |
| ChemSpider | 53492 |
| DrugBank | DB14099 |
| ECHA InfoCard | 03b2a12e-7e56-4aef-b6a4-c24109e7f7d4 |
| EC Number | 203-993-5 |
| Gmelin Reference | 82119 |
| KEGG | C19580 |
| MeSH | D015738 |
| PubChem CID | 8293 |
| RTECS number | TY2000000 |
| UNII | 79WB103J7S |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C10H22O4 |
| Molar mass | 190.27 g/mol |
| Appearance | Colorless liquid |
| Odor | Mild ether-like odor |
| Density | 0.951 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.96 |
| Vapor pressure | 0.045 mmHg @ 25°C |
| Acidity (pKa) | ~16 (estimated, for the hydroxyl group) |
| Basicity (pKb) | 7.43 |
| Magnetic susceptibility (χ) | -6.06×10⁻⁶ |
| Refractive index (nD) | 1.418 |
| Viscosity | 3.8 cP (25 °C) |
| Dipole moment | 2.26 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 259.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -726.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4680 kJ/mol |
| Pharmacology | |
| ATC code | D07AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 88°C |
| Autoignition temperature | 287 °C |
| Explosive limits | No explosive limits found. |
| Lethal dose or concentration | LD50 oral rat 3,960 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4,180 mg/kg (rat, oral) |
| NIOSH | RN 25498 |
| PEL (Permissible) | 100 ppm (TWA) |
| REL (Recommended) | 25 ppm |
| IDLH (Immediate danger) | Unknown. |
| Related compounds | |
| Related compounds |
Dipropylene glycol methyl ether Propylene glycol methyl ether Tripropylene glycol Tripropylene glycol monomethyl ether acetate |