Chemists first started exploring glycol ethers in the early twentieth century, drawn in by their solvent properties and low volatility. Triethylene glycol methyl ether grew out of this quest as a product designed for applications where water and oil solubility merged. Engineers and researchers in major chemical firms pushed for novel solvents throughout the 1960s and 1970s, eyeing efficient production and safer handling for workers. Those efforts led to the adoption of triethylene glycol methyl ether in coatings, inks, and cleaning industries, using more refined synthetic routes for improved purity and predictability. These days, newer regulations and sustainability goals are directing more projects toward alternatives, but this compound’s development track records the shifting priorities of the sector: better safety, precise application, and reduced environmental burdens.
Triethylene glycol methyl ether stands as a clear, colorless liquid with a faint, almost sweet odor. Companies pack it in drums and IBCs, labeling it for its distinctive combination of water solubility and mid-level boiling point. Customers with process chemistry backgrounds prefer its high solvency for tough residues in resins and dyes. Across its supply chain, you’ll spot labeling that references both the CAS number (112-35-6) and the name ‘Methyl Triethylene Glycol Ether’ to clear up confusion for purchasing departments and transport teams.
Measured in the lab, triethylene glycol methyl ether weighs in with a molecular weight of 178.22 g/mol. Its boiling point clocks around 255°C, significantly higher than its relatives like butyl or ethyl glycol ethers. The flash point hovers close to 120°C, making it less prone to accidental ignition compared to lighter ethers. It dissolves in water and most organic solvents. Its viscosity, density, and vapor pressure make it an easy fit for liquid blending, but not for spray applications needing rapid evaporation. Teams working with this compound lean on these numbers each day to build safety plans and production recipes.
Manufacturers post detailed specs, usually listing purity over 99%, maximum acidity values, and moisture content below 0.2%. Every container carries batch numbers for traceability. Labeling features international hazard pictograms, but old-school industry hands rarely rely just on packaging—most swear by regular in-house purity checks using gas chromatography, to guarantee that each delivery meets the expected technical grade before use in downstream products.
Synthesizing triethylene glycol methyl ether starts with the reaction of triethylene glycol and methanol under acidic or basic conditions. Operators choose catalysts and adjust pressure for maximum yield, then follow up with a round of distillation and purification to reach that much-needed chemical grade. In the past, glass-laden distillation setups dominated small-scale labs; most large plants moved on to continuous reactors, where engineers tweak residence times and temperature ramps for higher throughput and reliability. Any side streams with off-spec materials run through reprocessing to avoid loss and environmental fines.
This ether can undergo methylation or further ethoxylation to produce heavier analogs with altered solvency. Certain downstream industries react it with acids to form esters or with halogenated reagents to generate intermediates for specialty polymers. Process chemists like its flexibility and willingness to play along with general ether chemistry. Years of experience in paint and ink labs show its compatibility with both hydrophilic and lipophilic additives, a trait that keeps it in the toolbox when other solvents fail to mix.
While one product, it travels under many aliases: methyl triethylene glycol, methyl poly(ethylene glycol) ether, and some regional trade names. For ordering and cross-border logistics, relying on the CAS number clears up confusion caused by local name variants. Distributor catalogs list all synonyms to help procurement, but those in plant operations often stick with the shorthand “TriEGME”.
Safety teams treat triethylene glycol methyl ether with healthy respect—staff use gloves, goggles, and splash shields. MSDS documentation lists target organ risks, mostly kidney and liver based on long-term animal studies. Workers in production and repackaging monitor inhalation risks in poorly ventilated areas; engineering controls like LEV (local exhaust ventilation) remain non-negotiable. Spill kits contain absorbent pads and neutralizers specialized for glycol ethers. Plant managers run training sessions that drill these safety steps, knowing that a minor incident can become a regulatory headache.
Surface coatings and printing ink manufacturers buy it for its high boiling point and water compatibility. Electronics cleaning lines count on its solvency for removing stubborn polar contaminants. In pharmaceuticals, only the highest grades appear as tablet coating aids or trace solvents. Laboratory researchers value its predictability in organic synthesis. Industrial cleaners for heavy-duty equipment must cut through both grease and carbonized deposits, a trick that standard alcohols or simple ethers can’t always match. This solvent’s versatility stems from real-world experimenters looking for something that breaks the stalemate between water and oil.
Research teams keep combing through derivatives, looking to tweak toxicity, volatility, and clean-up performance. Some studies push for greener catalytic pathways; others chase after non-petroleum feedstocks. Data from environmental impact studies shape where labs focus next—either on making this ether safer or swapping it out for alternatives that tread softer on waterways and air quality. Regulatory bodies pressure researchers to lower emissions and lifecycle impacts, triggering a steady stream of new publications every year.
Animal tests set the stage: kidney and liver remain the main targets in high-exposure scenarios. Reproductive and developmental effects show up in summarized data, but not as strong as in more notorious glycol ethers. Acute exposure meets with headaches and nausea for workers skipping proper PPE. Chronic cases, while rare in controlled workplaces, keep popping up in less regulated regions, raising new calls for worker health surveillance and air monitoring protocols. Despite improved understanding over decades, skepticism about long-term low-dose exposure never really settles, prompting updated limits every few years.
Pressure for greener, safer solvents won’t let up. Some production lines eye biobased glycol ethers, especially where clients want biodegradable and low-tox products. Others focus on recycling solvent streams within plants, minimizing environmental load and raw material costs. Still, the core properties that made triethylene glycol methyl ether valuable—its strong solvency, balanced volatility, and blend compatibility—hold their place. Any replacement must match these traits or accept trade-offs. For engineers, chemists, and buyers, the search for safer, more sustainable answers follows direct feedback: what protects workers’ health, meets regulatory limits, and keeps processes running without costly surprises.
Some chemicals work quietly behind the scenes, moving from one factory to the next, never making the front page but holding everything together. Triethylene glycol methyl ether lands right in this group. If you haven’t heard its name before, you’re not alone. In industrial circles, folks call it TEGME. Wherever careful cleaning, electronics, or paints come into play, this stuff shows up.
My first run-in with TEGME happened way back in a university chemistry lab. We used it to wash bits of stubborn residue off glassware. A lab partner told me larger manufacturers use it for much bigger cleaning jobs. Textile mills often turn to TEGME as a solvent for dyes and inks, because it’s tough on grime without damaging fibers.
Electronics folks count on it when making circuit boards, especially if keeping water out matters. Unlike some older solvents, TEGME brings low toxicity at standard working concentrations. That sounds dry, but in practice, it means fewer headaches for the person on the line and less risk of fire breaking out.
On the production floor, TEGME fits into coatings and paints. It keeps paint smooth and workable, not gunky or streaky. Anyone who’s tried painting metal railings in spring humidity knows paints need help staying even. Formulators blend TEGME into water-based and oil-based products to slow evaporation and prevent ugly brush marks. In spray paints, this quality makes all the difference between a clean finish and something that flakes away after the first rain.
Printing shops lean into TEGME as well. Fast-setting ink poses a real challenge: you want quick results but hate the jams and blots that show up on high-speed presses. Recipes with the right dose of TEGME produce sharper prints, and that ripples down to cleaner labels, smoother magazines, and packaging that holds up through shipping.
People feel uneasy around chemicals, and for good reason. TEGME doesn’t rise to the danger level of many industrial solvents, but nobody should splash it around recklessly. Spending enough time with workers in factories or plants teaches you that safety measures like gloves, eye protection, and ventilation aren’t optional add-ons.
Research points to a lower risk of chronic human health effects compared to stronger ethers, but it still doesn’t belong down the drain or in backyards. Managing spills, recycling leftover blends, and following state environmental rules remain crucial. Some places have started piloting closed-loop recycling for industrial solvents, including TEGME. These moves save resources and cut down on the costs tied to hazardous waste disposal.
Nobody expects TEGME to disappear any time soon. With demand tied closely to electronics, paints, and coatings, steady demand will likely continue. The challenge falls on companies to keep refining how they use this solvent — making paints that last longer, inks that stay sharper, and workplaces that stay safer. In my experience, listening to the workers who use it every day brings more practical solutions than any whitepaper alone.
Triethylene Glycol Methyl Ether shows up in specialty industries—found in coatings, cleaners, and sometimes in labs. Folks I know in manufacturing treat this stuff with a real sense of respect because skin contact and fumes aren’t something to ignore. Stories float around about headaches and skin irritation following exposure, so just reading instructions isn’t enough. Common sense paired with direct experience has taught many workers to gear up properly and never take shortcuts.
Factories I’ve visited always focus on fresh air. Keeping workspaces well-ventilated isn’t up for debate. Exhaust fans above benches and open windows can mean the difference between feeling fine and suffering from nausea. If fumes collect, concentration in the air rises fast, which raises health risks—this isn’t a rare event. I have seen people try to fix a quick spill without turning on fans, only to cough for hours later. Small actions, like using fans and fume hoods, go a long way and cost next to nothing compared to a hospital visit.
Many factories provide nitrile gloves, splash goggles, and shop aprons. Reason for this: a single splash can burn or cause rashes. Too many times, folks cut corners, thinking a thin T-shirt has their back—only to end up regretting it. These days, I reach for long sleeves, gloves that actually fit, and eye protection that wraps around, not just basic safety glasses. Shoes matter too; closed-toe shoes beat sneakers or sandals every time, since spills don’t announce themselves before they happen.
One lesson comes from a night shift I worked at a small plant. Someone poured solvents into a drinking bottle—everything went downhill from there. Clear labeling of bottles, tanks, and even rags kept near chemicals helps prevent these avoidable mix-ups. If you spot a spray bottle without a label, don’t risk it. Toss it or fill in the info immediately. Organization isn’t just for show; it saves lives and keeps coworkers safe, too.
Spills and splashes happen fast. Colleagues who know exactly where the eyewash station is—or have the fire extinguisher checked and nearby—move with a certain confidence. Rinsing skin or eyes right after exposure prevents nasty burns and long-term damage. Safety drills may seem boring, but after seeing a quick rinse stop a friend’s eye from swelling, I never treat those drills as busywork.
Some people see chemical disposal as the last step, but it deserves more thought. Dumping used solvents down drains causes bigger headaches—clogging pipes, hurting water systems, and bringing fines. Local hazardous waste pickups exist for a reason. A call to local waste management or even a web search usually points to the right place for safe disposal. I’ve watched small companies face shutdowns just for skipping this step.
Triethylene Glycol Methyl Ether doesn’t forgive carelessness. Small details—reading Safety Data Sheets, never eating or drinking near workspaces, cleaning up spills at once—these shape the way people work and stay healthy in the long run. No job finishes faster than someone ends up in the ER. Cost of gloves and extra labels doesn’t come close to what one serious accident can cost a family or team. Watching old-timers and learning from their habits changed how I work around this chemical—and I wouldn’t go back.
Triethylene Glycol Methyl Ether isn’t a name that pops up on lunch breaks, but it quietly shapes products and processes behind the scenes. The chemical formula for Triethylene Glycol Methyl Ether stands at C9H20O4. Each molecule stacks together nine carbons, twenty hydrogens, and four oxygens. Its molecular weight lands at 192.25 g/mol. This isn’t trivia for a pop quiz — these numbers affect how well it mixes, boils, dissolves, and transports itself through any manufacturing line.
I once worked in a packaging plant, and there was a strong demand for chemicals that behaved the same from barrel to barrel. Triethylene Glycol Methyl Ether’s formula points to its structure as a glycol ether, meaning you get both water-loving and oil-loving sides. In practice, this makes it a go-to choice for cleaning solutions, ink formulations, and coatings where stubborn grease or ink needs breaking down without leftover streaks. The exact molecular weight helps engineers dial in what evaporates fast or clings on a bit longer — crucial for paint jobs in humid summers or printers buzzing at high speeds.
Chemists don’t ask for the formula just for fun. Safety sheets live and die by that C9H20O4 — it flags what fire risk comes from fumes, what gear to reach for if you splash some, and whether it’s topping up a supply or searching a warehouse database. Forgetting a single atom can mean reaching for the wrong drum, and trust me, nobody wants a surprise during a tight production run. The wrong label can turn into an expensive lesson after a bad batch.
Triethylene Glycol Methyl Ether doesn’t have the kind of notoriety that other solvents do, but it still calls for respect. Its moderate vapor pressure makes careless handling an open invitation to skin irritation or headaches. I remember one night shift where a leaky fitting made a space smell sweet, almost like old antifreeze. No one panicked — we’ve all seen worse — but folks started coughing in unison. It was a wake-up call to triple check gaskets and keep those nitrile gloves close. Knowing its weight and structure meant the ventilation team had answers ready when maintenance arrived.
Companies face tighter regulations every year. We all want safer warehouses and less hazardous waste, but getting there costs money and time. Some shops have started using closed-loop systems, cutting down on worker contact and capturing fumes instead of just running a fan. Digital tracking of inventory — scanning barcodes tied to molecular weights and formulas — is now more common than hand-written logs. It speeds up audits and makes sure emergency crews know what’s sitting on the shelves.
Everyone in the supply chain, from chemistry grads to forklift operators, benefits from simple, reliable information. The chemical formula C9H20O4 and a clear molecular weight aren’t just details for a textbook. They shape every step from mixing buckets to reviewing shipments and handling emergencies. Fact sheets, safety posters, and inventory systems rely on these small but vital pieces of info to keep people safe and products consistent. Understanding the makeup of Triethylene Glycol Methyl Ether isn’t glamorous, but it grounds the realities of working with chemicals every day — and that translates directly to smoother, safer work for everyone.
Everyone in chemical labs knows the same uneasy feeling. You screw the lid on a jug of solvent, push it to a corner, then start wondering: How safe is it sitting there? This really matters for Triethylene Glycol Methyl Ether. This chemical is no household supply—its low vapor pressure and affinity for water give it special quirks, but the mistakes people make are often pretty basic.
Triethylene Glycol Methyl Ether won’t explode like gasoline, but don’t let that fool you. Its flash point offers some breathing room, yet fires break out too easily around careless workers and neglected storage rooms. At my old warehouse job, I saw how storage planning could go awry when shortcuts replaced common sense. A spill on concrete, a missing lid, a cluttered space—trouble comes quickly. One small leak, and fumes can build up in places you wouldn’t expect.
Here’s where storage can make or break safety. I always look for containers built for chemicals— dense polyethylene or steel drums with tight seals. An open-top bucket or mystery barrel sounds ridiculous, but those roll into storage rooms all the time. Pressure-tested, tightly sealed drums protect against slow leaks and evaporation. Some folks skip grounding, but static likes to build up on insulated drums. A lightning storm in a bottle isn’t how anyone wants to see their day end.
It makes sense to store solvents in a dry, ventilated room, away from sunlight, heat, and strong oxidizers. Triethylene Glycol Methyl Ether won’t break down right away, but sunlight and air can still nudge it toward forming peroxides over months. Even without combustion, peroxides can damage equipment or trigger alarms.
Stacking barrels shoulder to shoulder may maximize space, but it slows everything down during an emergency. Spaces between containers help air move and let anyone spot problems— condensation near a seal can mean an upcoming leak. Every time I see an unlabeled bottle, I picture my old manager going pale as we tried to guess what was inside. Clear labels, always in plain language, avoid those moments.
Storage corners also become trash heaps if nobody checks them. I've found mystery drums from past renovations, veteran workers guessing based on faded paint or dusty documentation. Routine checks with a simple clipboard cut down on risk and help you catch slow leaks or swelling lids.
It’s tempting to park every solvent in the same row. That’s an easy road to disaster. Triethylene Glycol Methyl Ether doesn't belong near concentrated acids, strong bases, or any source of ignition. Even slight contamination can set off chain reactions you never see coming. One time, I watched a new hire clean up a spill with an old rag. No eye protection, no gloves, and he carried the rag past other solvents on his way to the trash. People remember lectures, but they remember examples even more.
Better storage starts with basics: solid containers, good ventilation, frequent checks, and distance from incompatible substances. Even a small investment in training and storage gear cuts both cost and risk. Safety isn’t a checklist—it's about staying aware, picking the best tools, and never letting routine invite carelessness.
Triethylene Glycol Methyl Ether doesn’t roll off the tongue easily, but you’ll find it in a bunch of industrial and commercial products. It helps manufacturers mix things together, dissolves ingredients, and sometimes turns up in cleaning agents. I’ve noticed, in my own work, that people often treat chemicals like this one as pretty harmless thanks to a lack of headlines or recognizable warnings. It’s got that “under-the-radar” feel, which isn’t always a good thing.
I’ve spent enough time around labs and workshops to learn that protective gloves and decent ventilation aren’t just suggestions. Triethylene Glycol Methyl Ether belongs to the family of glycol ethers. These substances raise some red flags; certain cousins like ethylene glycol ethers have been linked to headaches, fatigue, and, over time, even damage to blood and organs if exposure gets out of hand. This information comes from studies by organizations such as the National Institute for Occupational Safety and Health (NIOSH).
Triethylene Glycol Methyl Ether hasn’t grabbed headlines for serious health scares, but it still irritates the eyes, nose, and throat. Extended skin contact can dry you out, and nobody looks forward to nausea or stumbling through a headache after breathing vapor. People working regularly with these chemicals should take that seriously, even if the product label seems tame. Chronic exposure risks don’t always shout—they often creep up over months or years.
The stuff doesn’t only stick to gloves and aprons. Spills slip into drains or catch a ride with rainwater. Some glycol ethers break down, but not all disappear without a trace. Animals and plants living near factories can get more than their fair share—studies show glycol ethers can build up in aquatic life. Government sources like the EPA say it’s smart to keep any discharge extremely low to protect fish and water quality.
I remember hearing from a friend who works in wastewater that the trace presence of solvents can throw off treatment balances. That’s an eye-opener for anyone who thinks small amounts don’t matter. We can’t always see where run-off goes, but it keeps moving—through streams, onto fields, into food webs. The impact isn’t dramatic like an oil spill, but quiet problems can still get big over time.
Rules about glycol ethers vary a lot depending on where the plant or workplace sits. In some countries, strict controls limit exposure and demand proper storage or disposal. In other places, the chemical doesn’t attract much official attention. I find that confusing, given what scientists know about long-term risks. These uneven standards put more pressure on employers and workers to look after themselves, hoping nobody overlooks the fine print on a material safety sheet.
Switching out glycol ethers for something gentler would help. Some companies already do this, often where worker health or eco-certification matters most. Improving ventilation, using personal protective equipment, and never shrugging off small spills go a long way for everyday safety. Regular training keeps everyone alert—it’s easy to let routines slip, but old habits cost more than new gloves. Waste disposal deserves serious attention, too. Pouring leftovers down the drain only multiplies problems downstream, quite literally.
People can push for updated rules and keep an eye on what happens around chemical plants. Open conversations about workplace health and chemicals should always stay on the table—waiting for problems before acting rarely works out well. My experience says it’s safer to stay skeptical and careful, even if the label claims “low hazard.”
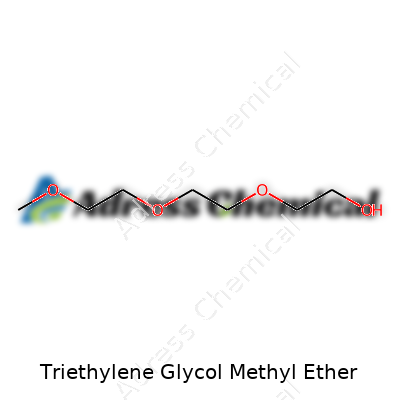
| Names | |
| Preferred IUPAC name | 2-methoxyethoxy)ethoxyethane |
| Other names |
1-Methoxy-2-(2-methoxyethoxy)ethane 2-(2-Methoxyethoxy)-1-methoxyethane TEGME Triethylene glycol monomethyl ether Methyl triethylene glycol |
| Pronunciation | /traɪˈɛθɪliːn ɡlaɪˈkɒl ˈmɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 112-35-6 |
| Beilstein Reference | 1771431 |
| ChEBI | CHEBI:34375 |
| ChEMBL | CHEMBL2057820 |
| ChemSpider | 12098 |
| DrugBank | DB14005 |
| ECHA InfoCard | 04eeed24-6c32-4f1a-9911-395c7f5c3379 |
| EC Number | 203-977-3 |
| Gmelin Reference | 1391736 |
| KEGG | C19605 |
| MeSH | D014272 |
| PubChem CID | 8183 |
| RTECS number | KL5950000 |
| UNII | T3748U896X |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID3021208 |
| Properties | |
| Chemical formula | C7H16O4 |
| Molar mass | 222.28 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild |
| Density | 0.995 g/cm3 |
| Solubility in water | Miscible |
| log P | -0.5 |
| Vapor pressure | 0.014 mmHg (20 °C) |
| Acidity (pKa) | 15.1 |
| Basicity (pKb) | 6.05 |
| Magnetic susceptibility (χ) | '-68.5×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.424 |
| Viscosity | 3.9 mPa·s (20°C) |
| Dipole moment | 3.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -885.65 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4076.7 kJ/mol |
| Pharmacology | |
| ATC code | D02AX07 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313 |
| Flash point | 121°C (250°F) |
| Autoignition temperature | 275°C |
| Lethal dose or concentration | LD50 (oral, rat): 6,600 mg/kg |
| LD50 (median dose) | 6,550 mg/kg (rat, oral) |
| NIOSH | RE1635000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Ethylene glycol Diethylene glycol Triethylene glycol Ethylene glycol monomethyl ether Diethylene glycol monomethyl ether |