Propylene Glycol Monomethyl Ether Acetate didn’t spring up overnight. Its roots run back to the surge of industrial chemistry in the 20th century. Back then, solvents that could dissolve both water-based and oil-based components held a lot of value in paint and coating manufacturing. The drive for higher quality, less toxic, and more efficient substances led chemists to focus on glycol ethers, especially those with better evaporation rates and lower odor. By synthesizing derivatives of propylene oxide, chemical engineers crafted a molecule that fit the needs of production lines: something with muscle but not as much smack as the usual solvents. This historical context matters as industries looked for safer, more reliable ways to boost performance without sacrificing the health of those working with these substances.
What stands out about Propylene Glycol Monomethyl Ether Acetate is its versatility. Producers market it as a colorless liquid that’s both a strong solvent and gentle enough for tasks requiring a lower toxicity profile. It ends up in printing inks, paints, and cleaners, filling a spot left open by earlier generations of harsh chemicals. Day-to-day in factories and workshops, techs appreciate that it doesn’t just do the job but also reduces the edge that comes with stronger, harsher solvents.
As someone who’s watched lab techs run routine tests, I’ve seen that Propylene Glycol Monomethyl Ether Acetate’s properties strike a balance. Its boiling point sits higher than typical ethers, around 146°C, making it less likely to flash off too quickly in paints and coatings. It mixes well with water and common organics, so it belongs on a shelf with go-to solvents. The mild, almost neutral odor is a small but significant upgrade for anyone who has ever choked on the fumes of classic cleaning fluids. The liquid flows easily, stays clear, and doesn’t leave streaks or residues, which has real benefits for folks doing finish work or high-end technical printing.
Manufacturers package it according to industry standards, including straightforward labeling: molecular formula C6H12O3, CAS number 108-65-6, and clear hazard warnings based on flammability and possible health effects. Tech sheets often detail density, viscosity, percentage water miscibility, and recommended storage. My experience in production environments showed how proper labeling cuts confusion and helps teams avoid mix-ups—a small but crucial safeguard against expensive mistakes and dangerous incidents on the line.
On the production side, chemical plants create Propylene Glycol Monomethyl Ether Acetate by acylation—reacting propylene glycol monomethyl ether with acetic acid or acetic anhydride. This method produces high yields with consistent quality, key for any process aiming to supply thousands of tons across global markets. Careful process control, from the purity of starting glycols to the tuning of reaction times, ensures the product meets tight specs. Knowing the process helps people on the job site understand what’s in their drums—and how any variation in raw materials or processing could affect end-use performance.
In the lab, chemists sometimes tweak or derivatize Propylene Glycol Monomethyl Ether Acetate to fine-tune evaporation rates, polarity, or reactivity for niche applications. Here, its structure gives flexibility. The ether and ester groups both open up different reaction routes. For example, under alkaline conditions, hydrolysis can slowly break the acetate back to the parent alcohol and acetic acid. In practical work, such knowledge is useful for recycling mixtures or troubleshooting situations where the solvent starts reacting with other chemicals in the batch.
People working with this solvent come across a long list of alternative names, most commonly PGMEA, 1-methoxy-2-propyl acetate, and sometimes the trade name Arcosolv PM Acetate. Each region and company may print a slightly different name on the label, but all point back to the same general formula. For suppliers and end-users, keeping track of synonyms avoids costly procurement errors.
OSHA and other regulatory bodies set strict standards on how this chemical gets handled. Exposure limits—typically set around 50 ppm—reflect a balance between industrial necessity and worker safety. Operating in facilities where this solvent is stored or transferred, I saw how easy it is for accidental spills or careless handling to cause problems. Practices like proper ventilation, protective gloves, fire-control measures, and clear spill procedures keep risk to a minimum. Training staff and tracking air concentrations should never feel optional but a crucial part of any operation.
Field techs and process engineers use Propylene Glycol Monomethyl Ether Acetate across a wide range of industries. In electronics, it serves as a photoresist remover. In paint and ink production, it delivers low streaking and superior pigment distribution. Factories making furniture or auto parts rely on its cleaning power and evaporation profile to leave surfaces residue-free and ready for coatings or adhesives. The breadth of application shows the solvent doesn’t just sit on a shelf as a backup, but as a daily workhorse in production environments.
R&D labs keep searching for ways to tweak formulae and improve mixtures that include Propylene Glycol Monomethyl Ether Acetate. The continued push toward water-borne and lower-VOC (volatile organic compound) coatings means chemists spend long hours testing alternatives and additions. In these labs, every improvement can mean less workplace exposure, stronger product performance, and quicker drying times—features that directly impact business margins and safety reports. From experience in chemical development, I see that balancing cost, availability, and environmental profile isn’t a linear equation—it takes dozens of lab trials and feedback from the field.
Toxicologists examine the impacts of repeated and long-term exposure to this solvent. While animal studies show relatively low acute toxicity, chronic exposure can cause irritation or more serious organ effects if not controlled. Workplace monitoring, ongoing health screenings, and detailed recordkeeping hold up as the best ways to ensure safety for those handling the chemical every day. Data show that Propylene Glycol Monomethyl Ether Acetate rates better than some huffier alternatives like toluene or xylene, but complacency can still trip up even an experienced handler. Keeping proper PPE and air monitoring front and center keeps people safer, which is always worth the extra step.
Demand for Propylene Glycol Monomethyl Ether Acetate won’t disappear as long as industries chase cleaner, safer, and more efficient production. Green chemistry initiatives could inspire newer, even lower-impact versions. Pressure remains to keep lowering VOC emissions and swap in renewable feedstocks. I’ve watched innovation happen when business leaders and chemists share data, trial alternatives, and listen to shop-floor feedback. If industry keeps attention on both worker health and environmental impact, there’s every reason to expect this solvent or its next-generation siblings to hold a solid place in many markets for decades ahead. Developing safer blends, closed-loop systems to reduce emissions, and tools to quickly monitor air quality will keep this chemical relevant, while sharpening industry focus on sustainability and human well-being.
Spend time in a freshly painted room and you might catch a slight whiff in the air that tells you industry has been busy. Paint doesn’t just magically flow from the bucket onto the wall; something needs to help it stay smooth and easy to spread. Propylene Glycol Monomethyl Ether Acetate (often called PMA) steps in here. It gives paints and coatings enough time to level out before they dry, avoiding the streaky mess that nobody wants to see on their living room wall. Without it, painting would feel more like a race against the clock.
Look inside your phone or computer and you’ll find a web of tiny parts all pressed together. Manufacturing those circuit boards involves materials that are tricky to handle. PMA finds its place on the production line as a cleaner and a process solvent. Electronics companies rely on it because it dissolves stubborn soldering residues and helps make those intricate printed circuits so reliable. Think about how many times you swipe across your screen or type on your keyboard; you expect these devices to work the same way every time. A clean and well-coated circuit board makes this possible.
Pick up a glossy magazine or a new paperback, and you’re seeing the results of more than just ink on paper. Printers use PMA to keep inks from drying too quickly, so every letter comes out crisp and clear. Without it, pages would be a smudged mess and images would look muddy. It gives printers and publishers enough flexibility to get things right, especially in large print runs.
Anyone who’s spent time working on cars or assembling auto parts knows grease and gunk aren’t welcome in high-quality finishes. PMA steps into the garage as a tool for cleaner painting on vehicle parts. Auto body shops and factories count on it to help finishes look flawless and stay that way after years under the sun and rain. Wherever vehicles need tough, good-looking finishes, this solvent shows up in the supply chain.
Using strong chemicals always brings up questions about health and safety. Breathing in vapors from PMA repeatedly, especially in workplaces with poor ventilation, can irritate your nose or throat and make you feel dizzy. Studies have shown that high levels of exposure for long periods aren’t good for humans, and workers need proper gear—good masks, gloves, and plenty of fresh air. The Centers for Disease Control and Prevention advises following safety guidelines for handling. Employers bear real responsibility to keep their people protected, not just to follow rules but because no job is worth trading for poor health.
There’s a conversation building in manufacturing about how chemicals like PMA could be swapped for safer options. Some industries look at water-based solvents and less toxic chemicals, though the transition is tough. Water-based coatings might need special equipment or take longer to dry, driving up costs and slowing down work. Factories can phase in machinery upgrades and hand out better protective gear while testing safer solutions. Eventually, industry leaders should step up the search for new materials that keep both performance and safety high.
Walk through a regular paint shop, car factory, or cleaning products warehouse, and you’re likely to run across Propylene Glycol Monomethyl Ether Acetate. This chemical pops up anywhere people want substances to dissolve, spread, or dry nicely. I’ve worked summers on construction sites and remember the sickly-sweet smell from fresh coatings — it’s easy to overlook the stuff inside the buckets and bottles that smooth out the work.
Breathing in the fumes for too long gives some folks headaches and a scratchy throat. A splash on bare skin, and you can get red patches or even a rash if someone’s sensitive. Office talk sometimes skips these details, but people in the middle of prep, spray, or clean-up see it up close.
Most people focus on paint fumes as an annoyance. Only deeper digging uncovers that propylene glycol monomethyl ether acetate (PGMEA) can cause more serious trouble with repeated, heavy exposure. Animal studies point toward nervous system and liver problems over the long haul. These aren’t everyday fears for the folks painting a fence at home, but the risks rise for anyone working with big batches indoors, day in and out.
Groups like OSHA and NIOSH pay attention to workplace exposure limits. The rule here is pretty simple: too much vapor in a closed room causes issues. Data from the National Institute for Occupational Safety and Health show that closed-up workshops with poor airflow let vapor levels rise fast. With symptoms as mild as dizziness or as tough as confusion and nausea, the risk builds over long shifts. Studies show that repeated exposure at high levels can put pressure on the liver and kidneys, organs that filter out the chemical from the body.
Practical experience tells me that companies cut the risk through basic changes. Even a cheap fan to keep the air moving and open windows makes a difference. Disposable gloves and goggles need to be on hand for anyone dealing with the concentrated liquid. I’ve seen places hand out material safety data sheets, but most workers learn faster with straight talk: don’t eat lunch at the bench, don’t smoke near open drums, and always wash up before heading home.
Manufacturers often shape the conversation, pointing out lower toxicity compared to old-school solvents like benzene. They have a point: PGMEA is less risky than some chemicals that used to be everywhere fifty years ago. Still, a lower level of danger doesn’t erase the need to pay attention.
A safe workplace owes its people the respect of honest information, clear labels, and working vents. The workers have skin in the game too — good habits make the difference between a bad headache and an easy shift. Sometimes government rules catch up only after someone gets sick. Nobody on the line wants to be first. That’s why small fixes, like fresh air and gloves, mean the most by the end of the week.
Propylene Glycol Monomethyl Ether Acetate—often shortened to PGMEA—shows up in paints, coatings, and cleaning solutions. I’ve walked through facilities where drums of PGMEA stack two or three high, and you can’t miss the faint, sweet, solvent smell. Simple as this liquid seems, a lot goes wrong without paying attention to storage and handling.
PGMEA evaporates fast and catches fire easily. I once saw an old storage area lit with incandescent bulbs and poor airflow—exactly the type of place that raises alarms for any flammable solvent. Companies face expensive downtime every year due to preventable fires and chemical leaks. That’s one reason why you keep PGMEA in a tight, cool space, away from spark sources. Welders and electricians who ignore warning signs turn small mistakes into bigger problems.
Steel drums or high-density plastic containers with tight-fitting lids last longer against spills and keep vapors from filling the air. Ventilated rooms help. I’ve heard seasoned warehouse workers remind new hires: “Don’t breathe it, and keep the fans running.” These aren’t empty words. Even a single unventilated closet can turn a minor splash into a headache for anyone nearby.
PGMEA doesn’t play well with heat. Warm storage makes it stink up the building and raises fire risk. Cold doesn’t hurt the product, but sudden changes in temperature create pressure inside drums. I’ve seen what happens when someone stores solvent barrels near a boiler or in direct sunlight—the drum lids bulge, contents spill, and the cleanup is a drag. Keep it out of these hot pockets; stable, moderate temperature keeps both product and people safe.
Once PGMEA leaves the storage area, things can get messier. Transfers between drums, tanks, or into smaller containers sometimes feel rushed. Spills, even small ones, call for quick action. I remember a coworker who always kept a stash of absorbent pads and gloves close at hand. Prompt cleanup stops solvent from soaking into concrete or trickling into drainage systems. Local fire codes usually stack high penalties on anyone careless about this step.
Solvent-resistant gloves and goggles do more than look official—they stop skin rashes and eye irritation. Training staff on how to handle PGMEA isn’t just a box to check; it protects everyone. Too many accidents stem from someone pouring too quickly, mislabeling a drum, or using an old, cracked line for transfer. Clear labels and up-to-date safety sheets go a long way in teaching even the most forgetful among us.
No one likes thick binders full of regulations, but regular safety walk-throughs help spot early leaks, rusted drums, or poor practices before they cost time or money. Automated leak detectors or alarms, once expensive, have become more affordable. Good lighting and visible signs make a difference. I’ve met warehouse supervisors who keep incident logs; reviewing these together—without blaming anyone—usually steers experienced teams away from repeated mistakes.
PGMEA doesn’t call for high drama; it asks for steady routines and respect for its properties. The basics never change: keep it sealed, keep it away from sparks, and clean up spills as soon as they happen. Better storage and handling mean fewer headaches—and safer workplaces.
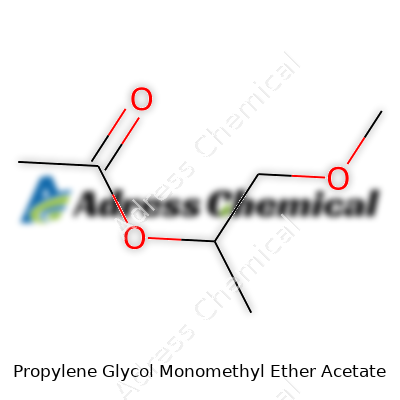
A lot of chemicals come with mouthfuls for names, and Propylene Glycol Monomethyl Ether Acetate is no stranger to that. Most folks in labs or factories just call it PGMEA. For those of us who need the facts without all the jargon, here’s what matters: PGMEA’s chemical formula is C6H12O3, and its CAS number is 108-65-6. These are more than just numbers or letters to plug into a database.
From the first day I poked around a college chem lab, the little white bottles with long lists of letters started to blur together. Still, it became clear that a chemical formula goes beyond the alphabet soup on a sticker. These six carbons, twelve hydrogens, and three oxygens aren’t just abstract calculus—this is the fingerprint that lets us track down exactly what’s in the bottle, so nobody plays guessing games. People can trust the safety data, source the right stuff, and stay out of trouble with regulators.
The CAS number, 108-65-6, gets used like a chemical’s social security number. Global suppliers and safety officers rely on it. Skipping it in procurement leads to headaches, maybe even shipments of the wrong solvent. In the real world, a slip in a number means ruined product batches or, even worse, dangerous situations.
I’ve worked alongside painters who unwrap cans after long days at construction sites. Much of what’s in those cans isn't just pigment, but chemicals doing the heavy lifting. PGMEA serves as a solvent in paints and coatings, especially where a clean, streak-free finish counts. Its formula lets it break down sticky stuff without dissolving every surface it touches.
In electronics, where clean takes on a whole new meaning, PGMEA has a regular spot in the production of semiconductors and printed circuit boards. Its mix of properties keeps delicate processes moving along, and you can count on suppliers needing more than a vague description when placing an order.
Safety can break down quickly if companies guess at what they are handling. Over the years, I’ve seen near-misses—wrong chemicals delivered, confusion over which substances needed special ventilation, reactivity surprises cropping up in the field. Knowing the right chemical formula and CAS number helps labs and factories draw hard lines between what’s safe and what crosses into risk.
Industries using PGMEA need airtight logs and labels. Regulations demand more than confidence—they require proof. The moment someone swaps even a single digit on a label, mix-ups happen that jeopardize quality and safety.
Training and clear documentation stand as the best guardrails. Anyone handling chemicals should have more than a vague sense of what those strange strings of numbers and letters mean. Audits help, but daily habits—triple-checking CAS numbers, confirming formulas—create the kind of workplace where people don’t have to rely on luck.
The core idea stays simple: treating Propylene Glycol Monomethyl Ether Acetate, or any chemical, as just another item on a shelf invites messes nobody wants to clean up. The chemical formula and CAS number remain critical, real-world guides—avoiding shortcuts keeps people and products safe.
Spills and chemical exposure don’t wait for a slow day on the shop floor. Propylene glycol monomethyl ether acetate, better known by its short form PGMEA, pops up in paints, inks, and cleaning solutions. This chemical sits on plenty of shelves but rarely in the public eye. Some employers and workers barely give PGMEA a second thought, but its fast-evaporating nature, irritating fumes, and skin effects demand a sharper eye and a practical routine for the real world.
PGMEA finds a spot on the list of chemicals that irritate eyes, skin, and lungs, even after a short encounter. Runny noses, red eyelids, or a rash don’t sound harsh, but skip over basics like wiping spills or using the right safety gear, and symptoms can get worse. Experience has shown that one overlooked spill on a concrete floor can lead to hours lost, or someone visiting the nurse feeling dizzy or wheezy. Facts from the U.S. National Institute for Occupational Safety and Health make clear: repeated exposure might eventually tangle with a person’s nervous system and long-term health.
Nobody welcomes a spill, but dragging feet after one happens just extends the risk. A quick approach helps—staff grab absorbent materials for liquids, toss on gloves and goggles, and keep air moving by cranking open a window or switching on the exhaust fan. Work stays simple: cover the wet patch, avoid creating clouds of vapor, and ditch any rags securely into proper containers. One person, not a crowd, steps up and takes control. After the mess disappears, mopping up with soap and water puts the last touches on the sweep.
No one enjoys guessing what they might inhale or what’s seeping through gloves. Workers appreciate regular lessons, not just posted signs, so training sessions where they can ask blunt questions or check how to wear a respirator make a real difference. It pays to check that spill kits sit in plain sight and everyone knows where fresh air vents or fans are hiding. Any label that’s tough to read or half-peeling loses its purpose.
Shops and labs that invite workers to speak up about missing gear or poor air flow build trust. Sometimes I’ve seen the best results when the leadership isn’t afraid to poke around on the floor, look into chemical storage cabinets, and get their own hands dirty in drills. Real safety shows through actions—walking the area, swapping out worn gloves, or maybe just asking if younger crew members actually understand the label instructions.
PGMEA won’t vanish from processes overnight. Still, manufacturers and supervisors who study safer alternatives or swap in lower-volume containers cut down chances for big spills. On a personal level, washing up before lunch, reporting headaches, or double-checking a label before mixing chemicals turns into a habit worth keeping. Industries slow to adapt often feel bigger accidents in the long run; motivating action almost always wins out over another memo buried in an inbox.
| Names | |
| Preferred IUPAC name | 1-methoxypropan-2-yl acetate |
| Other names |
1-Methoxy-2-propanyl acetate Propylene glycol methyl ether acetate PGMEA 2-Methoxy-1-methylethyl acetate Dowanol PMA Arcosolv PMA |
| Pronunciation | /ˈprɒpɪliːn ˈɡlaɪkɒl ˌmɒnəˈmiːθəl ˈɛθə ˈæsɪteɪt/ |
| Identifiers | |
| CAS Number | 108-65-6 |
| 3D model (JSmol) | `C[C@@H](OC)COC(=O)C` |
| Beilstein Reference | 1723807 |
| ChEBI | CHEBI:8811 |
| ChEMBL | CHEMBL1351066 |
| ChemSpider | 7472 |
| DrugBank | DB14006 |
| ECHA InfoCard | 100.017.781 |
| EC Number | Index No: 607-195-00-7 |
| Gmelin Reference | 1246359 |
| KEGG | C19609 |
| MeSH | D017561 |
| PubChem CID | 7915 |
| RTECS number | KL5950000 |
| UNII | 7GR28W0F1I |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C6H12O3 |
| Molar mass | 132.16 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Sweet ether-like |
| Density | 0.966 g/cm3 |
| Solubility in water | Miscible |
| log P | 0.43 |
| Vapor pressure | 0.49 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 14.8 |
| Basicity (pKb) | pKb: 15.2 |
| Magnetic susceptibility (χ) | -7.41×10^-6 cm³/mol |
| Refractive index (nD) | 1.400 |
| Viscosity | 0.43 mPa·s (at 25°C) |
| Dipole moment | 2.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 369.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3276 kJ/mol |
| Pharmacology | |
| ATC code | D02AE13 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | flame, exclamation_mark |
| Signal word | Warning |
| Hazard statements | H226, H336 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 2-2-1 |
| Flash point | 45 °C |
| Autoignition temperature | 315°C |
| Explosive limits | 1.5% - 7.0% |
| Lethal dose or concentration | LD50 oral rat 8532 mg/kg |
| LD50 (median dose) | LD50 (rat, oral): 8532 mg/kg |
| NIOSH | KVK87650 |
| PEL (Permissible) | PEL: 100 ppm (mg/m³) |
| REL (Recommended) | REL (Recommended Exposure Limit) of Propylene Glycol Monomethyl Ether Acetate: "100 ppm (540 mg/m³) TWA |
| Related compounds | |
| Related compounds |
Propylene Glycol Propylene Glycol Monomethyl Ether Ethylene Glycol Monomethyl Ether Acetate Ethylene Glycol Monomethyl Ether Diethylene Glycol Monomethyl Ether Acetate |