Propylene Glycol Monoethyl Ether, often flashing across chemical supply catalogs as PGME or 1-Ethoxy-2-propanol, didn’t pop up overnight. It owes its start to the rapidly growing synthetic chemistry era in the early 20th century. Companies working with propylene oxide first stumbled onto it while looking for new solvents that could cut through grease and grime. Production grew in response to the growing need for safer alternatives to harsher glycol ethers and improved cleaning power in both industrial and household formulations. A shift in how regulations viewed volatile solvents also nudged manufacturers to tinker with molecules like PGME. It became a staple for industries who needed something that helped break barriers between oil and water, carried fragrances, or cleaned without the choking smell trailed by some older chemicals.
PGME wears many hats. Manufacturers mix it into coatings to help paint glide and spread evenly before drying up. Ink makers like it for how it maintains fluidity during storage, but dries quickly when laid onto a page. Detergents and cleaners count on it for dissolving stubborn soils and oily residues from every kind of hard surface. It creeps into the formulating rooms of personal care labs, dissolving fragrances or helping alcohol blend into lotions and sprays. Everyday life keeps running up against PGME, but most folks never spot the name unless they read a safety label or material data sheet at work.
PGME comes out as a colorless, almost watery liquid, and most people can catch its faint but sweet ether-like smell if they lean close enough. It weighs less than water, with a boiling point that hovers around 120 to 135 degrees Celsius and slides off benches at room temperature with a viscosity close to water. The miscibility with water and many organic solvents makes it a chemist’s favorite for breaking up and carrying diverse substances. It flashes at about 39°C, so anyone handling it around sparks or heat keeps one eye on flammable gas policies. Its reactivity stays low under most storage and mixing conditions, but exposure to strong acids or oxidizers can stir up trouble—something chemical handlers watch with a practiced eye.
Producers set their specs around purity targets close to 99% for industrial and food-safe grades, keeping water, other alcohols, and glycols down to a tenth of a percent or less. Regulatory agencies call for clear labeling, including the hazardous substance code, flammability rating, and correct handling instructions. Unambiguous chemical names, batch numbers, and manufacturer details take up the rest of the label real estate, ensuring that workers, shippers, and customers know exactly what’s inside. Around the world, the demand for safety data sheets in the local language puts pressure on exporters to clean up any confusion before the barrels leave the warehouse.
Most PGME sits at the end of a process where propylene oxide and ethanol meet in the presence of a basic or acidic catalyst. Factories run these reactions in stainless steel vessels to dodge contamination and keep the process safe. After some time under heat and gentle pressure, the main product gets separated from any leftover reactants and water by distillation. The large plants—especially those in Europe and North America—invest in purification columns that clean the liquid until it meets demanding standards for purity, keeping customers’ processes running smoothly and shipping containers safe for transit.
PGME’s flexible structure lets chemical engineers tweak and change it for new jobs. Its ether bond resists most basic hydrolysis but gets clipped by strong acids, opening new routes to specialty intermediates. Companies sometimes build on the ether group, extending chain lengths or tacking on new functional groups. Production of esters from PGME isn’t rare either, finding use in fragrances or as low-volatility solvents in sensitive processes. Anyone who’s passed through an organic lab knows PGME pops up as both a solvent in reactions and a reagent to push chemistry forward, feeding into everything from simple cleaning agents to pharmaceutical intermediates.
Across catalogs, buyers spot PGME under a handful of names: Ethoxypropanol, 1-Ethoxy-2-propanol, and Propylene Glycol Ethyl Ether. In European chemical classification, it shows up as EC 203-804-1 and Cas No. 1569-02-4. Paint shops sometimes lump it in as part of “E Series Glycol Ethers,” an umbrella term that sends buyers straight to the technical pages to find the one that matches their formula lines.
Even for a chemical used in everything from ink to shaving cream, anyone handling PGME wears gloves and goggles and keeps extraction fans running. Inhalation or skin contact brings on eye, nose, and skin irritation. Short-term overexposure causes dizziness or headaches, so teams check ventilation and storage continually. Factories install spill kits rated for flammable liquids and train staff to mop up spills before vapors reach ignition sources. Labels and training materials keep accident rates low, and modern plants lock up stocks of PGME with fireproof doors and automatic suppression systems. The Occupational Safety and Health Administration (OSHA) and European Chemicals Agency (ECHA) both track exposure, and companies back up their safe practices with regular audits and on-the-ground drills.
PGME powers paint booths in auto plants and printing presses lining up today’s newspaper comics. Cleaning crews value it for its power to dissolve oily smears that plain soap won’t touch. Personal care formulations rely on it to blend scent and active ingredients, especially when alcohol won’t work or dries too quickly. Researchers test new pesticide formulations with PGME as a carrier. Industrial scale-up engineers reach for PGME to replace higher risk ethers in closed-system operations, lowering accident rates and cutting costs linked to hazardous waste handling. Its adaptability edges out older chemicals that either fail safety checks or lose ground when formulas demand more precision.
Labs chase down new applications for PGME every year. Chemists run trials on blending it with new polymers, searching for safer alternatives to solvents now flagged as hazardous by changing policies. Formulators push boundaries by tweaking concentrations, blending with cosolvents, and matching PGME with advanced surfactants. The academic side explores its impact on process efficiency, recovery rates, and purity outcomes in both established and emerging chemical synthesis. Research teams working with environmental agencies also probe how quickly it breaks down in soil and water, weighing its promise against growing sustainability targets. The push never seems to let up, as the pressure rises from end-users and regulators to do more with less environmental impact.
Workplace studies agree that standard industrial ventilation, full skin coverage, and short exposure times limit acute toxicity. Chronic exposure studies dig deeper, following workers for years to look for possible liver, kidney, or reproductive risks, but data continue to point to low bioaccumulation and a quick exit from mammalian bodies. The Environmental Protection Agency (EPA) flags moderate aquatic toxicity, pressing factories to close loops and prevent runoff into waterways. Community advocates still ask tough questions about air and groundwater risks, keeping PGME producers on their toes to prove safe handling in both big plants and small workshops. Regulatory groups and consumers alike keep pushing for tighter studies and clearer labels, especially as new evidence develops around alternatives and safer work practices.
The road ahead for PGME turns on evolving environmental regulations and green chemistry targets. Innovation in cleaning products calls for solvents that excel at lifting grease but break down faster in the wild, and PGME sits just on the edge between tried-and-true and up-for-review. Factory owners eye renewable feedstocks as the next frontier, hoping to swap petrochemical propylene oxide for biobased sources. Labs worldwide chase new derivatives with even lower volatility, pointing toward safer, less flammable options for tomorrow’s coatings and formulations. As public interest zeroes in on indoor air quality and safer work environments, PGME will either evolve to keep pace or step aside for new green contenders. The challenge isn’t just technical; it’s wrapped up in regulation, worker health, public trust, and the endless need to balance performance with peace of mind.
Most people have never looked at a list of chemical ingredients and thought, “Propylene Glycol Monoethyl Ether, that’s interesting.” But this long-named liquid shapes a pile of day-to-day products in ways few realize. Sometimes it hides behind a code, sometimes the whole name stands right there, but either way, you’re almost sure to cross paths with it more often than you think.
Growing up, I saw my dad working on cars or giving the living room a fresh coat of paint. He’d haul out cans, brushes, and bottles with warning stickers. Know what connected a lot of those projects? The presence of this solvent.
Paints, dyes, and coatings rely on Propylene Glycol Monoethyl Ether to spread color evenly and keep everything from separating in the can. Unlike thicker or harsher chemicals, this one mixes well with water and delivers a smooth finish without knocking you over with fumes. I’ve watched my old man toss rags and tools covered in paint, and the clean-up always came down to finding something that cut grease, lifted stains, and didn’t make the dog sick with the smell. This solvent checks those boxes for professionals and weekend warriors alike.
On the cleaning side, bigger companies use it to break up tough stains: homes, hospitals, mechanic shops, printers. Run your hand along a gleaming stainless steel table at a diner, and industrial cleaners with Propylene Glycol Monoethyl Ether probably wiped away the fingerprints that came before you.
Take a deep breath at your local bakery or spritz on cologne—chances are, a chemical cousin like Propylene Glycol Monoethyl Ether sits behind the scent. Perfumers and food processors use it because it’s good at dissolving and carrying flavors or fragrances without overpowering the original taste or smell. It plays a supporting role—never the star, but without it, you’d notice an odd, uneven hit of lemon in that pie filling or a perfume that smells pleasant for four minutes, then disappears.
Anyone who’s had to replace a dried-out inkjet cartridge or hose down a sticky label has run into this compound’s handiwork. It helps ink flow easily in printers and stops labels from gumming up in presses. Electronics manufacturers lean on it in circuit board cleaners because it doesn’t eat away plastics or delicate wiring.
That said, I can’t ignore stories I’ve heard from factory workers dealing with headaches or skin rashes, scrubbing down rooms at the end of their shift. While Propylene Glycol Monoethyl Ether is less aggressive than older, more toxic solvents, nobody wants to breathe it all day. Safety data shows chronic exposure at high levels can irritate eyes, noses, and lungs. I’ve found that updating ventilation, cutting down exposure times, and switching to gloves that don’t tear makes a difference for people who work closest with the stuff.
Waste presents another headache. Some plants collect and reuse their solvents. Others dump or burn leftovers, which can leak into the air or water. Local governments need pressure to enforce better handling and disposal, and companies respond to clear rules more than voluntary guidelines.
Progress comes with steady pushes. The market for “green” solvents grows year by year as companies experiment with replacements that don’t harm lungs or the planet. Some researchers look to plant-based chemicals or biodegradable mixtures. Using less and recycling more works too. There’s no simple path away from chemicals like Propylene Glycol Monoethyl Ether, but step by step, the world figures out how to clean, paint, flavor, and build smarter without leaving scars behind. A little vigilance—both from industry folks and consumers—drives that change further along.
Propylene Glycol Monoethyl Ether, known in some circles as PGME, pops up in many products. Paints and cleaning formulas rely on this substance to help things mix or clean more effectively. I’ve run into it plenty of times during work in the garage or while helping friends repaint apartments. On safety labels, it never hides. You’ll often spot a small warning on the back of a bottle—something about avoiding direct skin contact, eye irritation, or inhalation of fumes.
Folks tend not to pay much attention to chemicals unless something goes very wrong. Headlines love tough words like “toxic” or “carcinogen,” but PGME usually flies under that radar. Still, this doesn’t mean the risks vanish. The Environmental Protection Agency and the European Chemicals Agency both list propylene glycol monoethyl ether as a substance worth guarding against. They recommend gloves and plenty of ventilation.
I've seen too many people roll up their sleeves around solvents without a second thought. Months back, I watched a neighbor scrub paint out of his hair with a product containing PGME. Hours later, his scalp looked angry and inflamed. The stuff is an irritation risk, and the more you get on your hands, the more likely you’ll dry out your skin or trigger a rash. One way or another, every label deserves a quick scan before use.
Short- and long-term effects show up through the skin, eyes, or lungs. Inhaling high concentrations can mess with your respiratory system, leading to coughing, dizziness, or a nauseous feeling. Eye contact stings and can cause redness that lingers. Extended exposure, especially among folks who work in paint shops or janitorial jobs, sometimes causes headaches and fatigue, though most people won’t even recognize the source.
One crucial point: children and pets have lower body weight and faster breathing rates, and are at higher risk if exposed. I keep chemicals locked away for this exact reason. Once, a curious toddler I knew sniffed the open mouth of a cleaning bottle left out. It was a close call, so safety at home is not just about the adults.
An easy choice is swapping out bare hands for nitrile gloves. These provide a solid barrier against solvents and cost just a few bucks for a big box. I also crack windows or run a fan every time I’m working with anything that smells “chemical”—not just PGME. Opening the garage door or using a ventilator pulls vapors away and keeps the air you’re breathing clearer.
Keeping a pair of safety glasses in your toolbox solves the eye risk. It’s tempting to skip this step, especially for “just a quick task,” but I’ve felt the sting of a splash, and it’s not fun. I also make it a point to store containers tightly closed and up high—out of reach for kids or pets.
Calling something “safe” only works if the user respects the substance. My experience shows that most accidents come from ignoring instructions or shortcuts. Manufacturers already print common-sense guidance right on the package. With gloves, eye protection, and fresh air, the risk drops to a manageable level. The right habits outperform fear or risky bravado any day.
For people who spend time in industrial settings, I recommend checking Workplace Safety Data Sheets. These documents break down the risks in plain language and offer clear solutions—worth reading before pouring gallons out of a drum. Good training and simple gear go further than fancy labels.
Having spent time in a few warehouses and lived through more than one chemical safety briefing, it's clear that storing chemicals like Propylene Glycol Monoethyl Ether isn’t something you take lightly. This isn’t just another bottle in the corner. It pops up in cleaning products, coatings, and many industries for its solvent power. The thing is, nobody wants a leaky drum or a vapor that makes the air taste weird. Good routines help prevent slips, health problems, or product that’s gone off before using it.
Propylene Glycol Monoethyl Ether does best somewhere it can stay cool and shaded. Direct sunlight plays havoc on its stability over time, so placing containers away from windows or heat sources comes first. I’ve seen old warehouses rig up shaded awnings and fans after product losses taught them the hard way. Regular indoor temps below 30°C usually keep everything under control.
Ventilation matters. Anyone who’s popped open a chemical drum in a stuffy storeroom knows fumes linger. Decent airflow reduces unwanted vapor build-up and helps workers breathe easy. Someone working nearby will appreciate that open window or fan, believe me.
Moisture does these chemicals no favors. Damp floors risk corrosion and contamination. Rusted drums do not inspire confidence at inventory time. Simple plastic pallets or keeping stacks off bare concrete works wonders, saves trouble, and helps if a leak happens.
As for storage containers, original drums or approved poly containers work best. Make sure seals stay tight. If you ever come across container swaps or damaged lids, that's a red flag for contamination or evaporation. Regular checks—no skipping week after week—uncover problems before someone finds a sticky mess.
Gloves and goggles may seem like overkill to some, but it’s all fun and games until a splash hits your hand or eyes. I’ve seen new hires skip the gear to “just lift a drum” and regret it by lunchtime. Skin contact causes irritation, so nobody should handle it bare-handed.
Open up a container slowly. Controlled pour spouts and pumps beat quick tilts every time. A steady hand makes fewer messes and keeps odors at bay. If containers do leak, mop up using absorbent pads and steer clear of regular rags. Standard clothes carry the chemical around all day.
Mix-ups cause real problems. Labels matter, and double-checks aren't just bureaucracy. One wrongly labeled barrel and a line grinds to a stop, or worse, someone mixes it with something reactive. I once saw a team use color-coded tags and checklists to avoid confusion—simple, but it worked.
Drums shift in trucks and on forklifts. Secure everything with sturdy straps or blocks. Once saw a drum roll across a loading dock, leaving streaks nobody wanted to clean up. Steady hands, slow maneuvers, and watching routes saves headaches.
Disposing of Propylene Glycol Monoethyl Ether isn’t as simple as dumping it down the drain. Follow local hazardous waste guidelines, use dedicated waste containers, and consult professionals when in doubt. Communities do not take kindly to surprise chemical runoff, and fines can pile up.
At its core, proper storage and handling come down to clear routines, teamwork, and regular checks. Nobody can afford to cut corners. Discipline in the little things—labels, pallets, seals, gloves—saves money, protects people, and keeps businesses running smoothly. Investing in simple systems, training, and periodic audits closes gaps and strengthens safety culture. Every worker deserves that much.
Propylene glycol monoethyl ether isn’t just a tongue-twister. Working with it, you can’t miss its clear, colorless liquid form, along with a mild and almost sweet odor — not the sort of strong smell that lingers for hours, but one you easily notice in the air. This chemical mixes well with water and a range of organic solvents, which gives it some flexibility in real-world applications. If you’ve ever tried to clean old paint or worked in a printing shop, you might’ve handled it without knowing.
At room temperature, propylene glycol monoethyl ether stays stable, boiling at around 135°C to 145°C (275°F to 293°F). That’s high enough for most day-to-day use, yet low enough that you shouldn’t ignore open flames. Based on personal experience in a paint plant, I’ve seen this stuff go from a steady worker to a risky flammable liquid if safety protocols slip. The flash point, sitting close to 43°C (109°F), means just a little heat can make its vapors spark up. Keeping containers sealed and away from direct sunlight turns from basic advice into a must-do habit.
The magic of propylene glycol monoethyl ether comes from its dual nature: it jumps easily between water and oil-based solutions. This helps coatings stick smoothly, stops paints from drying too quickly, and makes cleaning up less of a headache. Surfactant properties — what lets it break up grease or stains — play a big part in household cleaners and industrial degreasers. People often don’t realize how silky a paint or cleaner feels until they switch to a brand without it. From my side, swapping out products missing this ingredient didn’t just change the color quality; it made cleanup a longer, messier job.
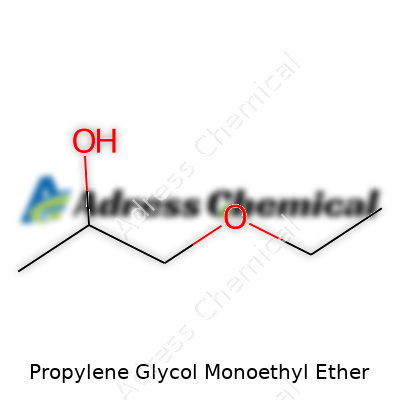
Chemically, the compound’s structure grants it these handy traits. Its molecular formula is C5H12O2. That balance of an ether and alcohol group means it dissolves both water-soluble and oil-soluble compounds. Its molecular weight of 104.15 g/mol might not mean much in conversation, but it tells formulators how it acts when mixed with other stuff. Because of this, it isn’t uncommon to spot this ether in printing inks and adhesives, playing middleman between substances that usually avoid each other. This bridge it creates can’t be understated for people looking to make efficient, smooth-running products.
Everyone who’s spent time in a manufacturing space learns quickly: don’t treat solvents like harmless props. Propylene glycol monoethyl ether can irritate skin and eyes if splashed. Prolonged exposure to vapors makes headaches and dizziness real risks, especially where ventilation lacks. In most shops I’ve worked, investing in simple protective gloves, goggles, and fans makes a clear difference. Rather than waiting for someone to get sick, training for correct handling and regular maintenance works wonders in keeping everyone safe. Pulling chemical data sheets out of a dusty drawer and making them part of the daily routine seems tedious, but it keeps surprises down to zero.
Finding greener substitutes grows as a hot topic. Some companies experiment with less volatile alternatives, yet few match the versatility of propylene glycol monoethyl ether without raising costs or complicating formulas. Regular safety audits and reviewing supplier information become a good habit — not just for ticking compliance boxes but for protecting workers. Big changes often start with simple, small steps: better labeling, more frequent inspections, or just listening when staff bring up problems. Clear communication in these settings means nobody feels in the dark, and that’s one way to make sure this chemical keeps serving its purpose without causing unnecessary headaches.
Propylene Glycol Monoethyl Ether—sometimes called PGME—doesn’t draw the same headlines as gasoline or ammonia, but its safety classification still matters for workers and communities. Many people brush by this topic because it sounds technical, but the way companies and governments treat PGME can shape real lives. If we turn our backs on what’s in the barrels moving down our highways and through our factories, we end up leaving the big decisions in hands far removed from our neighborhoods.
Anyone handling PGME wants a straight answer. The United Nations’ Globally Harmonized System says PGME can cause irritation if it gets in your eyes or sits on your skin too long. Some countries put it in categories where you’d find solvents—useful, but calling for watchful handling. The U.S. Occupational Safety and Health Administration (OSHA) doesn’t stick a “highly hazardous” label on it, but they still call for gloves, goggles, and fresh air if folks plan to work with PGME all day.
On safety data sheets, PGME tends to land in the “use caution” group. Eyes and skin can get red or sore, and with enough exposure, headaches and dizziness show up, just like with lots of other solvents. In high concentrations, it could start to bother the liver and kidneys. PGME doesn’t have a reputation for exploding or catching fire easily, making it less risky in transport than more flammable solvents. But the environmental agencies and transport regulators often lump it in with materials that need special paperwork and containers, if only so truckers and warehouse workers aren’t caught off guard.
For folks in small manufacturing, cleaning crews, or laboratories, knowing the difference between “hazardous” and “not hazardous” can change work routines. If a chemical counts as hazardous, it triggers strict storage rules, enforces limits on how much to keep on hand, and sometimes bumps up insurance rates for the whole shop. Workers might shrug at regulatory talk, but few miss the sting from accidental splashes or fumes. Even a chemical with a lower hazard grade becomes a problem if gear sits unused or fans stay off.
Community members don’t always get a say in what rolls through their town on a freight train. I remember working at a place where we used PGME as a cleaner. Any time someone read the label and realized it wasn’t some super-dangerous poison, you could feel a bit of relief—then see them leave their gloves in the drawer, trusting “not hazardous” to mean “harmless.” That’s a risky shortcut, and it proves how these labels shape behavior more than most folks admit.
Regulators and safety officers tend to play it safe, and for good reason. They draw lines based on years of accident reports. For PGME, cautious handling makes up for any gaps in classification. In my own work with solvents, I saw the best results from regular safety training, clear labels on containers, and steady airflow wherever solvents got used. Gloves, goggles, and a quick rinse station nearby saved more hassle than any paperwork ever did.
Some regions push for greener replacements wherever possible, since enough low-level hazards add up over time. PGME often gets swapped out for less irritating cleaners when budgets allow, but cost and performance keep it in rotation. If any policy deserves attention, it’s one that brings workers into the conversation and looks past just the label—because no chemical is risk-free, and no community wants to learn about hazards after something goes wrong.
| Names | |
| Preferred IUPAC name | 2-ethoxypropan-1-ol |
| Other names |
2-Ethoxy-1-propanol Ethyl propyleneglycol ether Ethyl-1-propylene glycol ether PGEE Propylene glycol ethyl ether |
| Pronunciation | /ˈprɒp.ɪ.liːn ˈɡlaɪ.kɒl ˌmɒn.oʊˈɛθ.ɪl ˈiː.θər/ |
| Identifiers | |
| CAS Number | 1569-02-4 |
| Beilstein Reference | 1361115 |
| ChEBI | CHEBI:8137 |
| ChEMBL | CHEMBL1658 |
| ChemSpider | 15354 |
| DrugBank | DB13955 |
| ECHA InfoCard | 100.017.886 |
| EC Number | 603-177-00-8 |
| Gmelin Reference | 6710 |
| KEGG | C19583 |
| MeSH | D026526 |
| PubChem CID | 8120 |
| RTECS number | KK8050000 |
| UNII | V444H4FN52 |
| UN number | UN3083 |
| CompTox Dashboard (EPA) | DTXSID5020652 |
| Properties | |
| Chemical formula | C5H12O2 |
| Molar mass | 118.17 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild ether-like odor |
| Density | 0.92 g/cm3 |
| Solubility in water | Miscible |
| log P | 0.23 |
| Vapor pressure | 0.67 mmHg (20°C) |
| Acidity (pKa) | 14.8 |
| Basicity (pKb) | 15.2 |
| Magnetic susceptibility (χ) | -0.56E-6 cm³/mol |
| Refractive index (nD) | 1.403 |
| Viscosity | 2.36 cP (25°C) |
| Dipole moment | 3.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -486.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3506 kJ/mol |
| Pharmacology | |
| ATC code | D07AX63 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1, 2, 0 |
| Flash point | 49°C |
| Autoignition temperature | 225°C (437°F) |
| Explosive limits | 1.5% - 12% (in air) |
| Lethal dose or concentration | LD50 oral rat 2420 mg/kg |
| LD50 (median dose) | 2,740 mg/kg (rat, oral) |
| NIOSH | UB4750000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Propylene Glycol Monoethyl Ether is 25 ppm (parts per million) as an 8-hour time-weighted average (TWA), according to OSHA. |
| REL (Recommended) | 25 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol monoethyl ether Propylene glycol monomethyl ether Propylene glycol monobutyl ether Diethylene glycol monoethyl ether Dipropylene glycol monoethyl ether |