Propylene Glycol Methyl Ether Propionate, or PGMEP, didn’t pop up yesterday. Its timeline winds back to the steady rise of demand for safer, smarter industrial solvents. Folks in factories and labs reached for alternatives to tougher, often hazardous, options. PGMEP found its stride in the 1980s as paint manufacturers and coatings experts wanted something that didn’t toss countless health risks into the air. The chemical world is always searching for ways to protect workers while keeping processes running strong. PGMEP feels like an answer from that era—one that grew up in the uncertainty of stricter environmental rules and the plain truth of occupational safety concerns.
You’ll spot this stuff not just as PGMEP, but as 1-methoxy-2-propyl acetate, PM Acetate, or Propylene Glycol Monomethyl Ether Acetate (PGMEA). Companies put their own stamp on it—Dow sells it as DOWANOL PMA, Eastman calls it Eastman PM Acetate. Regardless of branding, professionals want to know exactly what’s inside the drum before trusting it for the job.
PGMEP brings a clear, colorless liquid—few surprises at first glance. It carries a light fruity smell, sometimes almost sweet. Anyone who’s worked with solvents recognizes that scent on their clothes. Its boiling point sits around 145°C, and the flash point measures near 45°C. Not the most volatile, but it keeps users on their toes. Water and many organic solvents mix in easily, which lets PGMEP slide into cleaning and mixing without drama. Over the years, this ability to dissolve both polar and non-polar compounds turned PGMEP’s flexibility from a benefit to a reason for its continued use in industrial paints and inks.
Every barrel, drum, or container comes with a label that stays loaded with info: CAS Number 108-65-6, purity usually above 99%. Labels warn about flammability and urge proper ventilation. Looking closely, you see clarity standards, moisture limits under 0.1%, and acidity down near “trace.” The packaging is not just legal formality—it’s a message to staff that this is flammable, needs keeping away from sparks, and can hurt with excess exposure. I’ve always checked those details before tapping a barrel, since relying on guesswork in a warehouse just leads to trouble.
Synthesizing PGMEP asks for methyl etherification of propylene glycol, followed by esterification with acetic acid or acetic anhydride. The science behind it proves straightforward for the practiced hand but certain steps can trigger runaway reactions or produce byproducts—not a job for shortcuts. Plant engineers know temperature fluctuations and precise metering matter, right down to the kind of mixing blade in use. Small miscalculations bring on yield losses, which then hit the bottom line. In my time, problems usually trace back to inattentive control over reaction rates and purity of starting chemicals.
PGMEP doesn’t just sit tight as a solvent. Chemists subject it to hydrolysis, sometimes for recycling in paints, breaking it down to methoxypropanol and acetic acid. Skilled folks also tweak the propylene glycol backbone or switch out the methyl group, looking to fine-tune evaporation rates for printing ink or automotive coatings. I’ve seen shops experiment with blends using PGMEP as a base for stronger, faster-drying solvents, all trying to hit that sweet spot of speed, coverage, and safety on the shop floor.
Daily work with PGMEP calls for more than gloves and goggles. Proper fume extraction in painting booths and sealed storage tanks keep dangers out of airways and pipes. Spraying coatings means checking that fire suppression systems function, since flammable vapors can linger, especially on humid afternoons. I’ve watched old-timers skip respirators, but folks who stay in the business long enough learn to trust the Material Safety Data Sheet. PGMEP’s not acutely toxic, but can irritate skin, eyes, and lungs. Factory training pushes everyone to respect eye-wash stations and spill kits, not because rules say so, but because one incident leaves a memory—and sometimes a scar.
PGMEP stretches across industries—as a solvent in paints, coatings, inks, cleaners, and some adhesives. Automotive paints rely on it for the right balance between drying time and finish quality. Print shops value its low odor and quick evaporation, since slowing down a press isn’t an option. Manufacturers of electronics and semiconductors go for PGMEP in photoresist formulations, since residue spells defect risks. Many cleaning fluids count on its solvency to strip grease and contaminants, particularly before surface prepping for welding or painting. From hobbies to handheld devices, the touch of PGMEP covers more than people realize.
Industrial chemists look closely at toxicology and environmental impact. Studies show that PGMEP breaks down in the atmosphere, but not instantly—its half-life sits between several hours to days, depending on sunlight. The chemical doesn’t bioaccumulate, which calms some nerves, but human toxicity studies highlight symptoms like headache, nausea, and skin irritation at high vapor levels. Chronic exposure leaves questions, especially in crowded or poorly ventilated workplaces. Environmental teams keep monitoring air and wastewater, since regulations force companies to prove they’re not simply dumping solvents. Teams of researchers tackle reformulations just to keep levels of volatile organic compounds low, ahead of tightening laws. In my own time running process reviews, I’ve seen engineers and chemists spend late nights extrapolating from rat inhalation studies to set worker safety levels, hardly a glamorous pursuit but a necessary one.
PGMEP faces pressure from green chemistry advocates and tighter environmental rules across the globe. Paint firms and electronics manufacturers want less hazard on site and more sustainable life cycles from raw materials to waste handling. Biobased solvents threaten the market share of PGMEP, and companies lean into development work on alternatives that promise reduced emissions and easier recycling. Still, industries stick with what works, so major shifts take years, not months. I see research labs driving up purity levels and customizing evaporation rates more than before. Circular economy ideas push for recovery and reuse, meaning tomorrow’s plants may reclaim PGMEP from exhaust streams before anything escapes. Trade partners hope for consistency, lower cost, and compliance—so the days of slapdash substituting feel numbered. If new breakthroughs deliver on lower toxicity and better environmental profiles, the workhorse role of PGMEP will look a lot different from the one it serves today.
Step into a paint shop or a printing facility and you’ll probably smell something sharp in the air. Sometimes it’s the leftover tang from solvents like Propylene Glycol Methyl Ether Propionate, often shorted to PnB or PGMEP. This stuff does real work behind the scenes in modern industry, though hardly anyone grabs a can and reads the fine print on the back of paint or ink barrels.
Most folks want paint that dries fast, spreads smoothly, and looks good for years. PGMEP helps make that happen. It’s a solvent, which means it helps break down other ingredients—say, resins or pigments—so they mix well and form that glossy look on walls, metal parts, or fresh-print magazines. Factories value it for its strong ability to dissolve tough substances that otherwise clump up or leave streaks.
Printer shops also run into similar troubles. Thick ink gums up presses and leaves uneven lines, so PGMEP thins things out just enough to keep printing sharp without ruining the paper. It evaporates at just the right rate, meaning colors don’t bleed together but also don’t dry up on the roller before hitting the page.
Not all is rosy when workers handle strong solvents day in, day out. Breathing vapors over long shifts—especially in spots with poor ventilation—leads to headaches or, worse, more chronic issues like asthma. There’s the risk of skin irritation too. It’s not widespread public concern, but workers who spend hours next to open barrels or cleaning spray guns catch the brunt of it.
Regulations push businesses to use gloves, fume hoods, and lower-exposure formulas. In countries paying close attention to workplace safety, companies have swapped out the roughest ingredients for safer picks. PGMEP isn’t usually among the most harmful, yet it reminds us that chemicals designed to make life easier can still bite back if people cut corners.
The market’s impatient. Customers want cars painted yesterday, magazines printed last week. Fast-drying, streak-free properties make solvents like PGMEP hard to ditch. Water-based alternatives exist, but those setups need lots of tweaks, more expensive machinery, or extra drying time. Factories haul in loads of paint and ink every day, and the balance between productivity, cost, and safety isn’t easy to strike.
It’s easy to ignore what keeps our finishings sharp, but knowing these chemicals matter. Transparency in labeling gives a fighting chance to spot trouble before it hits. Workers should push for regular safety training and working gear that actually fits, not just token gloves. Research into safer solvents deserves more than a half-hearted budget, as smarter tech and stricter safety rules could reduce health complaints on the factory floor.
Every painted sign, glossy ad page, or coated tool in a hardware shelf likely passed through a stage where PGMEP showed its value. Instead of treating it as invisible, we should recognize the burden carried by the folks handling the stuff every day—and look for ways to lighten that load.
Many folks see a chemical name like Propylene Glycol Methyl Ether Propionate and their eyes glaze over. It sounds like something out of a sci-fi story, but it’s actually a solvent that shows up in paints, coatings, inks, and cleaning products. The big question on the job or in the garage—can you handle this stuff safely, or is it just an accident waiting to happen?
Here’s the truth: Propylene Glycol Methyl Ether Propionate (often shortened to PGMEP or PM P) isn’t the worst thing in the chemical lineup, but it’s hardly harmless. It slides off the tongue in safety meetings because it’s been used for years in industrial settings. Still, that doesn’t mean you should take it lightly. The U.S. EPA has information on it and the European Chemicals Agency does, too. They agree on one point—it can irritate skin and eyes, and it shouldn’t be inhaled in heavy doses.
During a stint at an auto refinishing shop, I saw two kinds of folks—the careful ones with gloves and goggles, and the ones who thought they’d be fine. The ones with red hands and itchy eyes were always in the second group. If you’ve ever been around the sharp tang of solvents, you know the feeling: a burning in the nose, watery eyes. It’s not going to kill anyone in one whiff, but over time, the story can change.
It’s easy to take shortcuts on ordinary workdays. Gloves slow you down and goggles fog up, but the price for skipping those steps never seems worth it after a trip to the doctor. PGMEP evaporates fast and can sneak into the lungs if you’re spraying or using it in closed spaces. Long-term exposure leads to headaches, nausea, or worse. Some studies in animals link repeated high exposures to nervous system effects, but people rarely end up around it for that long—unless safety gets ignored.
What actually helps on the shop floor or in a warehouse? Good old-fashioned commonsense. Always grab a pair of nitrile gloves, not those clear plastic food-service gloves. Ensure a window is open, or set up an exhaust fan. Don’t use it in a room barely bigger than a closet. I’ve learned not to trust fresh air just because a door is cracked. A proper respirator isn’t overkill, even if most people roll their eyes at them.
The law says employers should train staff about these risks, but too many cut corners and hope for the best. From what I’ve seen, workers talk to each other faster than management, so sharing tips and stories keeps people safer than a sign taped to the wall. It helps to keep a jug of clean water or an eyewash bottle on hand for quick rinses if there’s a splash. Spilling a solvent feels like a mess, but quick action can keep a minor problem from turning into a real medical emergency.
Technology, regulation, and workplace habits change, but one thing stands still—chemicals don’t care if you’re in a rush or having a bad day. My takeaway: treat Propylene Glycol Methyl Ether Propionate with respect and you’ll probably finish the week without regrets. Treat it like water, and the odds shift against you. Safety doesn’t need to be a chore. It needs to be a routine.
Anyone working in a lab or factory that uses Propylene Glycol Methyl Ether Propionate, or PGMEP, knows the job doesn’t stop at getting the chemical. What happens after delivery shapes everything from safety on the floor to the final product quality. This isn’t just another solvent; it’s flammable, has a smell that sneaks up on you, and likes to evaporate if you let it. So, storing and handling it with some thought really matters.
I’ve walked through quite a few storage rooms over the years, and every seasoned handler keeps the same checklist in the back of their mind. With PGMEP, the rules exist for a reason. A cool, dry, well-ventilated warehouse works best. Stashing the solvent in the shade, like under a sturdy overhead or inside a temperature-regulated space, keeps it from breaking down or leaking vapors out into the air. If you’ve ever faced headaches or dizziness after working with volatile organics, you know how quickly poor ventilation turns into a real health risk.
PGMEP likes to meet metal about as much as water likes old iron. Only high-quality stainless steel or tightly sealed HDPE plastic containers make sense. I’ve seen small leaks cause headaches more than once—spilled solvent, fumes in the air, routines thrown off. Even a forgotten open drum can start problems. Keep containers straight and above ground level where forklifts and palettes won’t bang them around.
You can smell PGMEP if the container isn’t fully sealed, and that should ring alarm bells. As with any chemical falling under the flammables list, never park it next to oxidizers or open flames. Smoking and open heat sources stay off the table completely. Sprinkler systems, proper signage, and dedicated fire extinguishers should never gather dust. I’ve watched drills where people forget which extinguisher to use, so regular safety refreshers keep everyone sharp.
The fewer people handle PGMEP, the better. Limiting access helps stop careless mistakes. Always grab the proper gloves—nitrile or neoprene over latex—plus splash goggles. One week without goggles is all it takes for new staff to realize their value, and rinsing your eyes in the eyewash is not a fun lesson. It’s smart to always keep spill kits at hand because dripping just a bit of this liquid on porous flooring makes cleanup a lot more complicated. Labels on drums help new folks just as much as veterans; labels fade, so check and relabel on a schedule.
Disposing of leftover solvent requires more than just chucking it in the bin. I’ve seen companies fined for dumping PGMEP down drains. The law says wasted solvent turns into hazardous waste. Pair it with a registered collection company and keep transfer containers closed tight until pickup. For the team, spill drills aren’t just red tape. Practicing with absorbent pads and neutralizers means less panic and better control if something goes wrong.
Following the manufacturer’s guide makes a lot of sense, but walking the floor and talking to the crew about real-world issues catches mistakes early. Regular audits and quick, practical training sessions keep everyone tuned in. Messy storage, sloppy drum stacking, or lazy labeling all invite trouble down the line. So the smartest move, from what I’ve learned, is staying humble—stay alert, keep it clean, and don’t let routine breed complacency.
Talking about chemicals can sound dry to anyone who doesn't spend time in a lab or on a manufacturing floor. Propylene Glycol Methyl Ether Propionate, often shortened as PGMEE Propionate, means a lot if you work in paints, inks, or coatings. Its chemical formula is C7H14O3, and you’ll often see people refer to its CAS number: 76913-02-1. Behind those numbers and letters lies a solvent that's built a solid reputation for reliability in both industry and daily life.
I’ve seen teams in industrial settings swear by this stuff because it handles jobs that regular solvents just can’t do as safely. In the coatings world, there’s always a struggle to balance drying times, safety, and how paints or inks spread. Everyone looks for solvents that tick more boxes: less odor, effective thinning, safer exposure levels, and environmental compliance. Propylene Glycol Methyl Ether Propionate brings that balance.
Workers often appreciate it for its low volatility. No one likes headaches on the job, and less evaporation means fewer harmful fumes compared to some old-school alternatives. Some solvents leave folks worried about hazardous air quality and lingering health effects. In the past, I've watched production lines slow down because compliance rules forced changes in formulas. Switching over to safer options like PGMEE Propionate helped keep those lines running and actually made the workspace better for everyone.
On the consumer side, anyone who's dabbled in DIY painting or used specialty cleaners may have experienced it firsthand—probably without knowing it. This solvent shows up in wood stains, specialty inks, and automobile finishes. Its good solvency and compatibility with both water and organic compounds make it a frequent choice in hybrid formulas. Most people want smooth application and durable results, and formulators rely on ingredients like PGMEE Propionate for that exact reason.
People worry about health and the planet more than ever. That's a point I respect, as I’ve seen stricter regulations hit fast and hard. The solvent world got a wake-up call decades ago about volatile organic compounds (VOCs). No one wants to breathe harmful fumes or see a solvent leaching into groundwater. Propylene Glycol Methyl Ether Propionate stacks up better than older, more toxic options—less smog formation potential and a better safety profile when workers are around it all day.
Still, using any chemical responsibly means more than switching one for another. Training remains vital. Proper ventilation, label understanding, and solid engineering controls go a long way. And even a safer solvent deserves careful handling—don’t let a lower toxicity rating breed complacency.
Safer chemical use becomes possible with clearer labeling and transparency. Sometimes, I notice small manufacturers or contractors struggling to get straight answers from suppliers. The industry could do more to bridge the education gap: easy access to safety data sheets, plain-language training sessions, and responsive helplines. Each one counts for reducing risk and boosting confidence.
Broader adoption of PGMEE Propionate shows that a push for balanced chemistry—effective, safer, and better for the environment—pays off. Success lies in responsible sourcing, continued worker education, and listening to the people who use these products daily. That, more than anything, will decide which chemicals become staples in industry and which fade out.
Walk into any hardware shop and the options for paints and coatings can make anyone's head spin. People don’t ask how a can of paint covers so smoothly or why that billboard print resists smudging day after day. Behind those finishes and clear graphics, there’s a quiet player: Propylene Glycol Methyl Ether Propionate, known in labs and on spec sheets as PGMEE or PGMEP. This isn’t some chemistry buzzword. Its performance and safety profile set it apart for some very practical reasons.
PGMEP is a clear, nearly odorless liquid. In practice, it offers a slower evaporation rate compared to basic alcohols or acetates. Anyone who has painted a wall or printed a brochure knows the drag of streaks, dry spray, and ink that clogs a head or dries before it hits the paper. PGMEP can stretch the drying window just enough. It’s not so slow that you’re waiting for hours, but it avoids the panic of a roller drying out mid-stroke or ink gumming up the works.
From a formulator’s standpoint, this makes PGMEP a reliable pick when consistent film formation matters. Its solvency—think of it as the ability to dissolve solids and resins that make up paint or ink—lets industries cut back on harsher chemicals without sacrificing performance. People notice the difference: a smoother application, a cleaner cleanup, and often, fewer headaches from overpowering fumes.
There’s always a drive to go cheaper or stick with what you know. Plenty of products rely on basic solvents like toluene or xylene. They dry fast and cost less. But try painting a metal frame as summer humidity creeps up, or turning out a run of fussy, high-gloss posters. Right there, the weaknesses show: quick-dry solvents lift and separate, leaving uneven surfaces or blurred images, sometimes before you can finish one coat. The absence of slow, even drying can mean more waste and more rework.
PGMEP’s real-world trait comes down to balance. It’s tough on stains and resin, but gentle around heat-sensitive prints or surfaces that can’t take a beating. This approach isn’t without questions—its higher cost can put off some buyers, and it requires thoughtful storage. Yet, the safety standards sit on a friendlier side compared to some older, harsher options. The potential for lower VOC emissions also matters as cities clamp down on air pollution, making compliance less of a headache.
Anyone involved in paints and inks knows the finish is only half the battle. Growing pressure for workplace safety and cleaner air means chemistry can’t stand still. Some teams are experimenting with water-based systems and biobased alternatives, and PGMEP often finds a spot in blends that bridge the gap between old-school strength and modern demands.
Nothing replaces a careful mix, as anyone who’s had to scrub up a sticky spill will say. Using PGMEP, it often means longer open time for coatings, brighter reproduction in printed inks, and a noticeable drop in harsh smells that linger in small shops or print rooms. It’s not a silver bullet. Still, given the usual headaches and hazards of our daily products, its uptick in use speaks volumes about how the industry pivots to smarter chemistry—one gallon, one batch at a time.
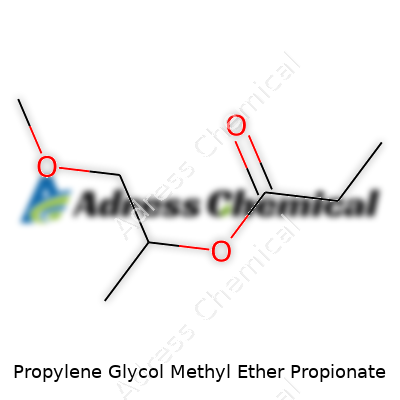
| Names | |
| Preferred IUPAC name | 1-methoxypropan-2-yl propanoate |
| Other names |
Propylene glycol monomethyl ether propionate PGMEP 1-Methoxy-2-propanol acetate Propylene glycol methyl ether acetate Dowanol PMA Arcosolv PMA |
| Pronunciation | /ˈproʊpɪˌliːn ˈɡlaɪˌkɒl ˈmɛθəl ˈiːθər proʊˈpɒneɪt/ |
| Identifiers | |
| CAS Number | Pn: 0056-70-3 |
| Beilstein Reference | 717276 |
| ChEBI | CHEBI:83090 |
| ChEMBL | CHEMBL1686086 |
| ChemSpider | 76694 |
| DrugBank | DB14238 |
| ECHA InfoCard | 05b1b496-7b24-452b-87fb-b80d41779948 |
| EC Number | # 484-120-7 |
| Gmelin Reference | 8576 |
| KEGG | C14389 |
| MeSH | D020089 |
| PubChem CID | 11429 |
| RTECS number | GO8925000 |
| UNII | 6G8W330V2T |
| UN number | UN 3272 |
| Properties | |
| Chemical formula | C7H14O3 |
| Molar mass | 160.20 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Faint odor |
| Density | 0.965 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.7 |
| Vapor pressure | 0.5 mmHg @ 20°C |
| Acidity (pKa) | Propylene Glycol Methyl Ether Propionate has a pKa of approximately 15.1 |
| Basicity (pKb) | 7.8 |
| Magnetic susceptibility (χ) | −8.80×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.404 |
| Viscosity | 1.2 mPa·s (25 °C) |
| Dipole moment | 4.9 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 152.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -576.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3070 kJ/mol |
| Pharmacology | |
| ATC code | D01AE15 |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Autoignition temperature | 287 °C |
| Explosive limits | 1.5% - 9.5% |
| Lethal dose or concentration | LD50 Oral Rat: 5,195 mg/kg |
| LD50 (median dose) | 6,500 mg/kg (rat, oral) |
| NIOSH | RN 7064 |
| PEL (Permissible) | 100 ppm (TWA) |
| REL (Recommended) | 100 mg/m³ |
| Related compounds | |
| Related compounds |
Propylene Glycol Methyl Ether Propylene Glycol Propylene Glycol Monomethyl Ether Acetate Ethylene Glycol Methyl Ether Dipropylene Glycol Methyl Ether Propylene Glycol n-Butyl Ether Propylene Glycol Phenyl Ether |