Propylene Glycol Methyl Ether—known by many chemists as PGME—has traveled a path shaped by the tide of industry and shifting scientific curiosity. Tracing its roots back to the push for safer, more effective solvents during the twentieth century, PGME started making waves as technology left behind older, riskier hydrocarbons. Waves of environmental awareness in the seventies and eighties spurred engineers to re-examine piles of solvents in their routines, prompting interest in alternatives that wouldn’t choke workers or catch fire at the drop of a match. Chemical companies started investing money and lab time into propylene oxide derivatives, looking for molecules that could thread the needle between strong solvency and manageable hazards. Chemists, including myself, have watched PGME step out of the shadows of its predecessors, finding a place in bustling workshops, print shops, and ever-changing paint factories, while regulatory agencies kept tuning standards for workplace safety and environmental stewardship.
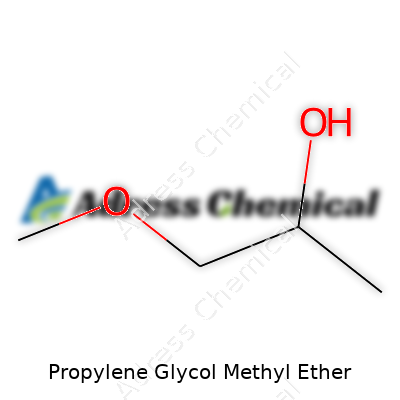
What exactly is PGME? Folks often picture it as a clear, slightly sweet-smelling liquid that gets poured from barrels in many different industries. This molecule, with the formula C4H10O2, sits among the so-called glycol ethers—compounds prized for their ability to dissolve greasy substances yet disappear quickly into the air. In my own work, PGME stood out for its balance: strong enough to break up inks, greases, and resins, yet not so harsh that it wrecks equipment or gives users headaches. Many art supply stores, printing houses, and auto-body shops stock PGME as a workhorse cleaner and thinner. Over the years, I’ve seen it help strip paint and lubricate machines, proving resilience in the face of competitive alternatives like ethylene glycol ethers.
PGME behaves predictably, and this reliability draws users looking for consistent results. At room temperature, it stays liquid, with a boiling point near 120°C, so it evaporates neither too fast nor too slow—making it handy in both quick-dry jobs and longer evaporation runs. PGME mixes well with water and many organic solvents, meaning one product can handle a big variety of tasks without too much fuss. Its vapor pressure and flash point offer enough safety margin for most applications, but not so sluggish to gum up equipment or leave residue. I’ve handled plenty of batches myself—always appreciating the lack of a strong, choking vapor cloud and the way it rinses clean from glassware and tanks.
Tough regulations mean the label on a drum of PGME reads like a miniature novel: purity above 99%, moisture content snuggled below 0.3%, and acid numbers in the low decimals. Most suppliers deliver it in steel containers with clear hazard warnings, reassuring end-users that they're getting the right grade for their needs. Safety Data Sheets usually come stapled right to the paperwork, outlining flammability warnings and first aid steps the moment the seal cracks. Over the years, specifications have tightened as equipment sensitivity increased, so users now expect more transparency from suppliers before they bring a batch onto the shop floor.
Factories manufacturing PGME implement a simple, robust route—a reaction involving propylene oxide and methanol under controlled conditions. This process yields substantial amounts of valuable product with relatively few byproducts. On the plant floor, I've seen technicians keep a watchful eye on reaction temperature and feed rates, because runaway conditions can result in unwanted impurities or introduce waste. Catalysts fine-tune the reaction, and purification steps remove water and excess alcohols, ensuring barrels ship out filled with reliable solvent the next day.
Versatility sits at PGME’s core. Labs have explored its role not just as a solvent but as a starting point for other molecules. PGME can react with acids or halides, forming new esters and ethers. In one memorable project, colleagues used PGME’s sturdy backbone to build custom surfactants for specialty ink formulations, opening doors for tailored products in niche markets. These downstream modifications hinge on trusted reactivity and predictability—qualities users crave in bulk commodity chemicals.
Anyone sifting through catalogs will spot PGME’s long list of aliases: 1-Methoxy-2-propanol, Dowanol PM, and Propasol PM, among others. Navigating this landscape gets tricky, as regulatory filings and technical bulletins sometimes use different names for the same stuff. Having handled orders for companies big and small, I learned fast that clear product tracking and proper documentation matter just as much as purity and price when shipments cross borders or auditors show up.
Talk about safety, and every workplace veteran sits up straight. PGME isn’t odorless, but its relatively mild scent makes it less likely to set off complaints. Like all glycol ethers, it carries risks: inhalation of high vapor concentrations can cause drowsiness or headaches, and skin contact deserves respectful handling procedures. From my earliest days stacking shelves, I heard horror stories of ill-ventilated workshops—so the sight of PPE, local exhaust systems, and updated safety plans acts as reassurance. Key standards spelled out by OSHA and the European Chemicals Agency require proper storage, use of goggles and gloves, and fire mitigation steps in case a spark turns minor mishaps into emergencies. Training new workers about flammability and exposure limits can feel routine, but the alternative—accidents or chronic health issues—proves the wisdom of those reminders.
PGME earned its keep smoking out oil stains, thinning paints, and clearing away resinous build-up. In automotive shops, it helps dissolve fresh paint, giving a smooth finish that resists blemishes; printing presses use it to keep ink systems running clean through big runs. More than once, companies running old equipment reached for PGME to replace forbidden glycol ethers, satisfied by the results and manageable regulatory hurdles. In electronics, PGME shuttles between cleaning and wafer processing, prized for its gentle touch paired with consistent solvency. The range stretches further—manufacturers of household cleaners, agricultural chemicals, and adhesives all tune their blends for this versatile solvent.
Chemists in corporate and academic labs keep digging for new uses and safer alternatives. Recent years brought mixed questions: can PGME take over roles from older, more toxic solvents? Can it handle new environmental pressures as governments keep lowering permissible exposures? I've watched research teams build experimental protocols now required before any new glycol ether reaches industrial scale—studies on vapor release, breakdown pathways, and long-term worker health. Some labs run projects on blending PGME with co-solvents for low-VOC paints, with startups making noise about all sorts of sustainable chemistry approaches. The challenge grows: how to balance tradition and innovation in an industry that prizes both predictability and progress.
Health impacts take center stage in regulatory hearings, and PGME’s record stands up better than older glycol ethers. Animal studies reveal modest drops in toxicity, but persistent use or prolonged exposure can lead to mild central nervous system effects—headaches, nausea, or drowsiness in poorly ventilated spaces. My own early days in the lab hammered home the lesson: don’t brush off safety, even for “less-toxic” chemicals. Chronic exposure limits exist, enforced by governments wary of long-term cancer or fertility risks linked to similar molecules. The evidence still accumulates, but in practice, companies reduce exposure with proper engineering controls, turning what used to be a wild west into a safer workspace.
As demands change, PGME faces a gauntlet of shifting goals: stricter safety standards, tighter emissions caps, and constant competition from bio-based solvents drawn from renewable crops. In the field, users still appreciate the reliability and relatively low health risks, but research groups push for “greener” tweaks—lower toxicity derivatives, faster biodegradation, cleaner downstream processing. With each new study, companies re-examine how to blend tradition with the urgent call for sustainability. I’ve sat through heated debates at industry conferences, where every old hand and enthusiastic scientist weighs in on whether PGME’s golden years roll ahead or if the search for safer, more sustainable molecules turns the page on another industrial staple.
Anyone who has ever taken a look at the ingredient label on a can of paint or a bottle of household cleaner probably glossed over words like Propylene Glycol Methyl Ether (PGME). That’s not surprising — most of us aren’t chemical engineers, but this ingredient makes a difference you can see and sometimes smell on any freshly painted wall or perfectly polished surface. You won’t find it front and center in ad campaigns, but it shows up every day in real life. Factories rely on it for its strength as a solvent, businesses like it because it keeps products consistent, and people benefit from surfaces that look smoother and last longer.
Construction workers and DIY enthusiasts battle with streaky, patchy paint more often than they’d like to admit. Painting a room without trouble means working fast and keeping the finish even. PGME steps in to dissolve pigments and binders so coatings flow evenly and don’t leave clumps of paint behind. Brushes and rollers stay cleaner because this solvent doesn’t evaporate as quickly as something like acetone. That slower evaporation lets you cover a bigger area without those dreaded lap marks. I’ve seen it firsthand on a jobsite—cheap supplies without the right solvents end up showing every flaw on the wall.
Anyone who’s tried scrubbing a tough mark off their favorite table knows some stains won’t lift with plain water. PGME works in many household and industrial cleaners because it tackles inks, greases, and stubborn marks that other ingredients can’t touch. Many manufacturers switched to PGME when they started phasing out stronger, harsher chemicals over health concerns. The compound’s lower toxicity level and quick breakdown in the environment keep it in the mix for safer cleaning fluids, especially around food prep areas or in schools where kids touch every surface.
Thumbing through a glossy magazine or checking out the crisp graphics on a box, it’s easy to forget all the steps that turned blank paper into eye-catching images. Commercial printers need inks that dry fast but won’t clog their machines. PGME balances out the formula to get that ideal drying time. Too fast, and ink dries in the press; too slow, and pages stick together. It even helps inks hold color longer under sunlight, another win for the shelf life of anything from cereal boxes to catalogs.
Questions about chemical safety come up over and over. Nobody wants mystery fumes in schools, homes, or workplaces. PGME’s track record, compared to the tough solvents it replaced, leans in its favor. That doesn’t mean it’s perfect. People with asthma or sensitivities sometimes need extra ventilation or personal protective gear. In my own shop, I learned to trust the material safety data sheets, never just the claims on the container. Regulations push the industry to keep developing safer blends, and worker training takes the guesswork out of using new formulations.
PGME often sails under the radar, yet delivers real benefits. From easier-to-clean schools to more durable finishes on vehicles, it shapes daily life in ways most people never consider. Keeping focus on transparency, safety precautions, and continued innovation can help it play its part—quietly but effectively—in making our surroundings cleaner, safer, and longer-lasting.
Propylene glycol methyl ether gets a spot in a lot of workplaces. You see it in paints, coatings, cleaning products, and printing inks. The promise is smoother formulas and surfaces that dry better and quicker. In my years working with industrial products, hardly a week goes by without bumping into this chemical. The question of safety sticks with plenty of people, especially those who use these items day in and day out.
Each time someone asks about its safety, I look to data first, not marketing claims. Well-documented studies tell us this chemical isn’t as notorious as heavy hitters like benzene or toluene. Research shows the body breaks it down pretty fast. If you breathe in a little from the air in a well-ventilated shop, your body gets rid of it soon after. Skin contact, unless it’s splash after splash, rarely leads to major trouble.
Regulatory boards in the US and Europe have given it the green light for use in many consumer products. OSHA and the EPA post exposure limits—25 parts per million in workplace air. Working inside those limits, most healthy folks don’t face outlandish risks. It helps that its smell gives an early warning before levels get too high. Many painting crews already trust their noses to tell them it’s time to crack a window.
Problems pop up mostly in small, closed rooms. I remember one workplace where ventilation didn’t measure up, and paint crews started reporting nausea and headaches. They powered through, but it made clear that just because a chemical seems “safe,” you can’t shrug off protective steps. One day makes little difference, but working with it five days a week, 40 weeks a year, builds up your risk.
Touching it, especially on cracked or damaged skin, sometimes brings out redness. Some users notice irritation in their eyes or lungs if they stay around it for too long, especially if the area stays stuffy and hot. So yes, safety goggles and good gloves play bigger roles than people guess.
Over the years, the safest shops dial into prevention. Good air flow cuts down on vapor buildup. Companies switch to water-based mixes when possible. They lean on manufacturers to supply clear information instead of fine-print labels. Training makes an even bigger difference—when folks know how to spot symptoms of overexposure, they stop problems before they turn serious.
For people at home, ventilation helps more than fancy air purifiers. Short bursts of use, open windows, and wearing gloves take most of the sting out of handling these products. It’s worth checking the safety sheet on any cleaner or paint, not just for scary chemicals but to see if anyone in the house has allergies or sensitivities.
Calling a chemical “safe” leaves out the bigger picture. Propylene glycol methyl ether isn’t a monster, but treating it with respect keeps it from becoming a hidden problem. Pressure sits on manufacturers and retailers to make sure labels give folks everything they need to know. At work or home, the basics—airflow, gloves, knowing your limits—carry more weight than fancy promises about “green” or “non-toxic” products. If something feels off while using it, don’t tough it out. Step out, breathe fresh air, and listen to what your body tells you.
Propylene Glycol Methyl Ether, or PGME, comes up a lot in conversations about solvents, printing inks, and paint strippers. I’ve seen workshops stocked with this chemical, where folks lean heavily on it for cleaning or thinning. The thing with PGME is it’s flammable and can cause headaches if ventilation is poor. Anyone using or storing PGME needs clear steps to keep things running smoothly and people safe.
Every workplace I’ve visited that relies on PGME invests in well-ventilated storage. Stuff PGME in a cramped, warm spot, and sooner or later, the vapors will hit you or, worse, find a spark. Industrial safety guides recommend storage areas be cool, out of direct sunlight, and away from heat sources like boilers or radiators. People sometimes get casual during busy days, slipping a drum of PGME near a maintenance room or an electrical panel “just for some time.” These quick fixes pile up risk. One spark and vapors can ignite.
PGME should sit in tightly sealed steel or HDPE containers. I remember a small manufacturer using old paint cans and plastic jugs to hold leftovers. The stench lingered, and lids didn’t close properly. Over a few weeks, leaks wound up causing headaches and some burned hands. Factory floors are no place for makeshift packaging.
People often think spills or burns just happen to careless workers. Most cases I’ve seen happen because someone ran out of gloves or rushed to finish before lunch. PGME soaks into skin easily and long exposure breeds health problems. To keep hands protected, nitrile gloves work best. Splash goggles are non-negotiable too, because any drop in the eye feels like fire. Clothes should cover arms and legs.
Pouring PGME straight from a drum never ends well. Spouts or pumps that attach securely keep things cleaner and reduce splash risk. Labeling helps too, especially with several clear-looking liquids lying around. Accidental swaps—using PGME instead of water or vice versa—have happened more than once in places I've worked.
Good airflow cuts down harmful concentrations fast. One paint shop I know set up fans facing away from workers, pointing straight at open doors, and it made a difference. Without flow, vapors collect, and even people not directly using PGME start feeling sick. Regular checks of vents and filters matter, since they get dusty and clogged in high-traffic settings.
Spills happen even with the best plans. Absorbent pads handle small messes. For larger spills, I recommend keeping sand or commercially available absorbent granules close by. It pays off to keep everything off the floor, store pads and cleaning kits where anyone can grab them, and post reminders on the wall so newcomers don’t scramble for instructions.
Ongoing hands-on training outshines dry lectures. People learn more by running through “what if” scenarios. Regular drills and refreshers build habits and catch blind spots before something goes wrong. In my experience, workers who practice together spot each other’s mistakes and encourage safer routines.
At the end of the day, safe work with PGME doesn’t rely on fancy tools—just good habits, clear labels, and respect for what the solvent can do if left unchecked.
Ask anyone who’s worked in paint shops, cleaning supply warehouses, or print rooms—they know the smell of solvents better than most. Propylene Glycol Methyl Ether, often called PGME, is one of those familiar names. Its chemical formula, C4H10O2, and the CAS number 107-98-2 might look like random figures, but they open doors to a world filled with practical uses and real challenges.
I remember painting my cramped apartment years ago and being handed two cans—one listed “propylene glycol methyl ether” on the label. The store clerk explained it makes paint smoother and easier to work with. He knew what he was talking about; the paint dried evenly and there were fewer unpleasant fumes. Turns out, PGME’s volatility and solvency power land it in a ton of household and industrial products: from glass cleaners to printing inks. It manages to dissolve greases, oils, and resins, making it the go-to for countless manufacturers.
Using chemicals in your daily routine sometimes means trade-offs. PGME doesn’t reek as strongly as some other solvents, but ignoring ventilation is still a recipe for headaches. While research out of regulatory bodies points out PGME generally raises fewer red flags than harsher cousins like toluene, it still earns respect. Short-term exposure can lead to dizziness or throat irritation. One thing that always sticks with me from factory training: Just because a chemical smells less intense, that doesn’t make it harmless. Reading labels and wearing gloves occasionally made me the odd person out, but I rarely missed a day of work for mysterious coughs or skin rashes.
PGME offers a gentle balance—its evaporation rate bridges that gap between fast-dry solvents and those that linger, making it popular for water-based coatings. Demand has ballooned as industries have pressured suppliers for safer ingredients with solid performance. The downside is the environmental pressure it creates. Spills seep into groundwater unless companies hold themselves accountable. Waste disposal hasn’t caught up everywhere, raising concerns about long-term health effects for communities near manufacturing hubs.
Growing up in a parts-cleaning shop, shifts left my clothes smelling like strong solvent by the end of the day. We’d talk about how the industry could use fewer toxic chemicals if alternatives worked as well. Now, green chemistry teams race to design cleaners and coatings with less harm in mind—there’s movement, but switching out PGME is no overnight job for factories deeply invested in their old processes.
One answer lies in education: Training workers and DIY users not just in product safety, but in smarter alternatives and proper disposal. On the regulatory end, greater transparency from producers and stricter pollution controls would nudge industry away from risky shortcuts. The right incentives and research will drive adoption of less hazardous choices. Progress always comes slower than hoped—for now, PGME continues to fill its niche, but there’s a growing push to place health and environment on equal footing with industrial convenience.
Propylene glycol methyl ether—PGME for short—shows up in plenty of places: cleaning products, paints, inks, and coatings, not just in big factories but on the shelves of ordinary stores. Lots of folks have never heard of it, but it helps keep things smooth, spreadable, and easy to apply. The thing is, many people might not realize the hazards that tag along with it.
Open a label. You might see warnings about flammability. PGME catches fire more easily than water evaporates. It gives off vapors that ignite if they meet flames, sparks, or even hot surfaces. That's not a small risk, even if you’re just storing a can in your garage or using a cleaning spray in a stuffy room. The National Fire Protection Association gives this chemical a flammability rating of 2, which means it can burn if things go sideways. Just a forgotten rag or a carelessly flicked cigarette lighter can cause real trouble.
Flammability isn’t the only problem. Breathing in vapors from propylene glycol methyl ether over long stretches—or in a space with lousy airflow—may lead to headaches, dizziness, or a feeling of fogginess. Once in a while, I’ve helped repaint rental apartments, and by hour three, people start complaining of sore eyes and scratchy throats. It’s hard to brush that off as just bad luck or cheap paint; the chemical mix plays a big role.
Contact with the skin might sound harmless, but it gets absorbed. Folks who work with degreasers or auto parts often find their hands dry and cracked from this stuff. The risk of irritation might seem minor at first, but over weeks or months, it turns into rashes or itchy patches. For some, even a short exposure can provoke an allergic reaction.
Too many companies and homeowners miss the hazards, partly because PGME doesn’t smell too strong or sting your nose like ammonia. Even with tighter regulations and data sheets, many workplaces let the basics slide—faulty lids, missing gloves, no fans humming in stuffy corners. I’ve seen corners cut when deadlines push people to hurry. Safety gear stays in the cabinet. The temptation is always there, and it never disappears on its own.
Worries about household and workplace accidents keep popping up, but real changes start with habits. If you storm through a repainting job, open windows wide. Block off sparks, turn off anything that could heat up nearby. Always store cans tightly and away from the heat. Gloves and goggles block most of the irritation before it starts.
Responsible companies swap in safer chemicals when possible or provide honest training, not just a quick slideshow. At home, read labels—don’t stash flammable supplies under the sink where pets or kids poke around. Ask questions at the hardware counter if labels look confusing. Folks shouldn’t need a chemistry degree to keep safe, but it makes a difference to take a moment and think about what’s in the bottle.
Looking for alternatives isn’t just about ticking boxes for safety. People deserve products that don’t trade a clean surface for a wheezing chest or burnt fingers. Shifting habits, both at work and at home, helps cut accidents. Common sense, clear information, and a little respect for the risks—these steps keep real people out of trouble.
| Names | |
| Preferred IUPAC name | 1-methoxypropan-2-ol |
| Other names |
1-Methoxy-2-propanol PGME Propylene glycol monomethyl ether 2-Methoxy-1-methylethyl alcohol Dowanol PM Arcosolv PM Methoxypropanol |
| Pronunciation | /prəˈpɪliːn ˈɡlaɪˌkɒl ˈmɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 107-98-2 |
| Beilstein Reference | 803153 |
| ChEBI | CHEBI:8185 |
| ChEMBL | CHEMBL16860 |
| ChemSpider | 6549 |
| DrugBank | DB03737 |
| ECHA InfoCard | 03-2119475108-42-0000 |
| EC Number | 200-923-1 |
| Gmelin Reference | 66844 |
| KEGG | C02328 |
| MeSH | D011380 |
| PubChem CID | 8093 |
| RTECS number | TY2000000 |
| UNII | 6DG9V0D43E |
| UN number | UN3092 |
| CompTox Dashboard (EPA) | DTXSID7020287 |
| Properties | |
| Chemical formula | C4H10O2 |
| Molar mass | 90.12 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Sweet ether-like odor |
| Density | 0.924 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.43 |
| Vapor pressure | 10 mmHg (20°C) |
| Acidity (pKa) | 14.8 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | −9.72×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.405 |
| Viscosity | 1.7 mPa·s at 25 °C |
| Dipole moment | 2.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -477.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2294 kJ/mol |
| Pharmacology | |
| ATC code | D02AX |
| Hazards | |
| GHS labelling | GHS labelling for Propylene Glycol Methyl Ether: `"Warning; H226; P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235"` |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 42°C |
| Autoignition temperature | 287 °C (549 °F; 560 K) |
| Explosive limits | 1.5 - 13.1% |
| Lethal dose or concentration | LD50 oral rat 5660 mg/kg |
| LD50 (median dose) | 6,010 mg/kg (rat, oral) |
| NIOSH | RN 107-98-2 |
| PEL (Permissible) | 100 ppm |
| REL (Recommended) | 100 ppm |
| Related compounds | |
| Related compounds |
Propylene glycol Propylene glycol methyl ether acetate Ethylene glycol methyl ether Dipropylene glycol methyl ether Propylene oxide |