Decades ago, industrial chemists took a hard look at the limits of conventional solvents and kept pushing for alternatives that could handle complex tasks in coatings, inks, and electronics. A compound that started grabbing their attention was Propylene Glycol Ethyl Ether Acetate (PGEA). This substance may not stir up headlines, but over time, experiments and real-world demand carved out a niche for it in fine chemistry labs and production facilities. Just as with many specialty chemicals, the story began with specialists tinkering in glass vials, chasing higher performance and cleaner handling. As electronics and automotive finishes kept evolving, so did the appetite for more stable and efficient solvents like PGEA, quietly edging out older, less versatile substitutes.
PGEA carries a reputation in the industrial world for being a colorless, almost odorless liquid that pours with a viscosity that reminds one of light oils. Many product datasheets might rattle off comparisons with ethyl glycol ethers, but PGEA's edge shows up in tougher environments where resins or specialty coatings call for both solvency and controlled evaporation rates. This solvent pulls its weight in more than one sector, showing up in cleaners, paints, and electronics. When I walked through a paint manufacturing facility, the barrels lined up with PGEA labeling told part of the story: a behind-the-scenes player that helps keep colors sharp and layers smooth.
PGEA doesn't just blend in with other acetates. It boils at temperatures ranging between 156-161°C and shows decent miscibility with water and many organic liquids. The substance brings together an ether and acetate group, which provides both a quick flash-off for drying and a moderate evaporation rate—details that matter quite a bit to formulators. Density usually falls near 0.96 g/cm³, and the refractive index settles around 1.417, which comes into play in certain optical coatings. Its flash point, usually right above 50°C, matters a lot for anyone handling drums or tanks, especially in warm climates or cramped workspaces. The chemical stability, combined with a resistance to hydrolysis under most application scenarios, helps PGEA earn a place in systems that can’t tolerate decomposition or gumming up.
Each drum and container needs to spell out more than just the chemical identity and batch number. Industry standards expect detailed purity assessments, often 99% or better, and tight controls on moisture and acid content. Regulatory marks—such as UN numbers, hazard pictograms, and specific phrases—show up on every label, not only to satisfy oversight bodies but to provide clear guidance for anyone storing, transporting or pouring the substance. From experience, it’s the missing technical data—like specific gravity or water content—that can trip up a production batch, causing off-spec results and headaches on the factory floor. Reliable suppliers make sure their paperwork lines up not just with national regulations, but with the everyday concerns of chemists and operational workers.
Getting PGEA out of the laboratory and into tankers usually involves a straightforward reaction between propylene glycol ethyl ether and acetic acid or acetic anhydride. This process brings in an acid catalyst and carefully controlled temperatures, often running in batch reactors with constant stirring. From what I’ve seen, skilled operators watch for signs of off-colors or excessive byproduct. Some proprietary tweaks to reaction times and purifications can raise yields above 90%, which matters a lot for economic reasons at scale. Purification takes center stage, with distillation and drying steps clearing out residual acids, unreacted alcohol, and water. Even a touch of impurity can foul up downstream applications, especially in high-tech uses.
PGEA’s structure leaves it open to some interesting chemical manipulations. The ester group, for one, allows for hydrolysis under strong acid or base, splitting the molecule into its alcohol and acid halves. That property isn’t always wanted, so most users shield the compound from harsh environments during storage and use. Chemically, its main appeal is not so much in what it transforms into, but in its ability to dissolve and interact with polar and non-polar resins. It can take part as a solvent in one-pot syntheses, helping other ingredients blend or react, but you don’t see it acting as a feedstock for bigger molecules very often. Still, a few research labs have tinkered with chemical modifications to tweak its evaporation or polarity.
Plenty of names float around in catalogs or shipment manifestos for this chemical. Besides Propylene Glycol Ethyl Ether Acetate, labels might say 1-Ethoxy-2-propyl acetate, EEP acetate, or even the CAS Registry Number 2370-98-5. End users navigating technical documents or regulatory filings bump up against all these terms. Distributors often slap on their brand names, which just adds to the confusion for folks ordering from multiple sources. The synonyms don’t hide any drastic differences, but they do underline the importance of double-checking formulas and safety info before mixing or substituting.
Dealing with PGEA means taking personal safety and facility standards seriously. Overexposure tends to hit the central nervous system and, in closed spaces or with poor ventilation, may cause dizziness or mild headaches. It evaporates slower than some short-chain solvents but still gives off enough vapors to require solid exhaust systems and, in busy settings, frequent air quality checks. I’ve worn gloves and splash goggles during decanting, since slight skin and eye irritation occurs if splashed. Local and national guidelines demand robust spill control, flammable storage cabinets, and clear signage. Firefighters and safety officers know the risks: open flames or static discharges near storage tanks remain an absolute no-go.
PGEA has made a quiet mark across several industries. In electronics manufacturing, it pops up in cleaning steps for delicate parts, where harsh alternatives would wreck sensitive materials. Paint technicians rely on it for high-gloss automotive coatings, where drying profiles and smoothness come down to fine details in solvent blend. Printing facilities often keep it on hand for ink formulations that need to deliver both clarity and setting speed. Occasionally, you run into it in specialty cleaners or adhesives, particularly where greasy or oily residues are involved. With all these uses, performance on the workbench decides repeat orders more than specs in the marketing brochure. The folks spraying panels or printing circuit boards judge value from hands-on results.
Labs continue to turn out new blends and recipes built around PGEA, chasing greener profiles or improved performance. University groups and private researchers aim for modified acetates that biodegrade faster or generate less hazardous waste. Over the years, government funding and industry partnerships have driven studies into better catalyst systems, more efficient production routes, and purification methods that chop water and energy use in half. A few startups have tackled PGEA reuse and recycling, looking to recover spent solvents from manufacturing waste and repurpose them instead of burning or dumping. The race for lower toxicity and higher efficiency means new derivatives and blends are constantly being tested in pilot lines before full-scale adoption.
PGEA’s safety profile isn’t all smooth sailing. Toxicologists have carried out animal studies and traced metabolic breakdown products, focusing on nervous system effects and longer-term impacts. Acute exposure causes mild irritation, but chronic inhalation or skin contact at higher doses yields liver and kidney impacts in test subjects. Regulatory watchdogs set workplace exposure limits and require updated Material Safety Data Sheets to flag emergent risks. Case reports from factory floors have prodded changes in ventilation requirements and personal protective gear recommendations. The push for less hazardous alternatives, especially in countries with strict chemical controls, keeps propelling continued toxicology research.
New regulations, stronger market demand for eco-friendly materials, and global drives for waste reduction will shape PGEA’s future. The chemical’s role in next-generation electronic devices and automotive finishes won’t fade quickly, but competitors fueled by biotech and green chemistry might start grabbing more market share if they deliver comparable results. From what I’ve seen, direct feedback from plant operators and research chemists will carry more weight than glossy product launches. Ongoing collaborations between industry and academia look crucial—innovations around process intensification, recycling, and hybrid solvent technologies stand to reshape the market. As clean energy and advanced manufacturing keep driving innovation, the compounds that blend performance with safety and sustainability will take center stage.
Propylene glycol ethyl ether acetate sounds more like a chemistry quiz answer than something you’d encounter outside a lab. Still, this stuff spends plenty of time in the real world. Walk into an auto body shop, or step into a room that’s just been freshly painted, and you’re already in its territory. It catches a ride in solvents, breaking down paints and inks so they glide on smooth and dry fast. Every product that demands a factory-fresh finish, from your car’s shiny fender to the printed label on a soda bottle, probably owes some sparkle to this compound.
When I helped a friend repaint his kitchen cabinets, streaky patches kept appearing, and nothing seemed to fix it until he mentioned a special cleaning and prep fluid. Many of these “secret sauce” mixtures lean on chemicals like propylene glycol ethyl ether acetate. Instead of gunking up a brush or leaving drips on the wood, the solvent thins paint just enough to give an even coat. That’s a lifesaver for industrial painters too, where time pressures mean a finish must look good on the first try.
Anyone who has run a business with a thermal label printer or printed posters for an event will know the pain of smudged ink and faded colors. Ink makers use this acetate to keep inks from drying out too quickly in the nozzle, but once the ink hits paper or plastic, it dries quickly. Fast, consistent drying means bold prints and fewer production halts. Digital printers depend on it, especially for flexography and gravure processes that print on flexible packaging—think snack bags and drink cartons.
Smartphones and tablets wouldn’t look half as slick if their components didn’t start off cleaner than your average dinner plate. Manufacturing plants use strong solvents to prep circuit boards, ensuring not a trace of dust or grease clings to the delicate wires. Propylene glycol ethyl ether acetate’s knack for dissolving greasy substances without destroying sensitive electronics makes it a go-to ingredient for precision cleaning.
This solvent works hard, but it also demands respect. Inhaling its fumes in a closed space over several hours can bring on headaches or dizziness. Factories install strong ventilation, and workers wear masks—because skipping safety steps isn’t worth a ruined workday. Even for do-it-yourselfers at home, a cracked window takes little effort and keeps headaches at bay. Regulatory agencies such as OSHA and the European Chemicals Agency pay close attention, constantly reviewing limits and recommending new ways to minimize exposure.
Many manufacturers hunt for alternatives that don’t sacrifice performance or safety. Some plant-derived solvents have started showing up in eco-friendly paints and inks. They’re not perfect, but progress doesn’t land in one giant leap. Until safer solutions become just as practical and affordable, propylene glycol ethyl ether acetate remains a workhorse across industries. What matters is balancing quality with conscious use, supporting new research, and not turning a blind eye to risks hiding behind tricky chemical names.
Many chemicals drift through daily conversation, mostly unnoticed. Propylene glycol ethyl ether acetate hides behind a long name, yet turns up in paint thinners, coatings, inks, and cleaners. Paint and ink manufacturers like it because it works fast and dissolves tough ingredients, so you might find it anywhere from renovation sites to print shops. But just because a chemical works well, doesn’t always mean it works safely.
Plenty of folks don’t recognize the technical name, but that doesn’t offer any protection from the fumes or skin contact. Propylene glycol ethyl ether acetate’s vapors can catch a sensitive nose right away—high concentrations burn the eyes, throat, and lungs. Short bursts might cause dizziness or headaches. Step into a closed room with no fresh air, and things get worse: heavy exposure can irritate the skin, and enough of it could slow your reaction time, maybe even trigger nausea.
Studying chemical safety, I’ve found it’s usually about the dose, route, and duration of contact. As with many solvents, using the product for short periods in well-ventilated spaces usually keeps risks low. Leave a container open in a closet, or splash it on bare skin day after day, and the chance of trouble jumps. Prolonged and repeated exposures could lead to skin rashes or long-term effects on the respiratory system.
Seeing “acetate” often causes confusion, since folks tend to lump it together with the much more famous acetate, ethyl acetate, which pops up in nail polish removers. Yet, propylene glycol ethyl ether acetate has a slower rate of evaporation, so its fumes don’t hit as hard or as quickly as some old-school solvents. Still, less doesn’t mean harmless—just ask anyone who’s walked out of a poorly ventilated garage, woozy from paint prep work.
Unlike some heavy industrial toxins, this chemical doesn’t show strong links with cancer or birth defects, based on published studies. The main risks concentrate on acute exposure, not subtle, sneaky long-term effects. But it pays to take even minor hazards seriously, given how many people carry out home DIY or help kids with art projects.
Common sense habits make a real difference. Open windows; use fans; never ignore the package instructions. I’ve seen too many cases where skipping those steps led to sick days or doctor visits that could have easily been avoided. Disposable gloves, masks, and keeping bottles tightly sealed cut back most of the direct risk. Many workplaces take it further, relying on workplace air monitoring and encouraging regular breaks from tasks involving strong chemicals.
Some manufacturers have moved toward greener formulas. Sometimes the safer alternatives don’t work as fast or cost a bit more. For jobs in small, enclosed places, nobody ever regrets choosing the safety route—especially after realizing that headaches and burns aren’t a badge of honor.
Painters, printers, and hobbyists shouldn’t live in fear of the chemicals around them, but knowing what you’re facing matters. Asking about product safety, searching for substitutes, or just cracking open another window brings better peace of mind than powering through with crossed fingers. Propylene glycol ethyl ether acetate has its uses, but respect and healthy caution go a long way. It never hurts to check the label and act before the smell fills the air. Better to pause and protect your health than rush and pay for it down the line.
Propylene Glycol Ethyl Ether Acetate (PGEA) enters the scene in coatings, inks, and cleaners. Most folks in the industry know all too well the sharp, somewhat sweet odor. Touching or breathing in too much of it can irritate skin and lungs, which means careful handling always takes center stage.
These chemicals don’t just sit in any old room. Direct sunlight will degrade it faster than expected, heating could spark pressure build-up in containers, and open air leads to moisture or oxygen creeping inside. A cool, dry, shaded spot makes the biggest difference. Warehouse managers who tuck drums or intermediate bulk containers away from windows and hot pipes see longer shelf life and fewer headaches.
Ventilation stands out as the next piece of the puzzle. Chemical fumes always manage to find their way out, and poor air flow often turns minor leaks into bigger problems. Over the years, I’ve learned a basic exhaust fan or open louver stops those breathing complaints before they start. It also gives workers more time to spot a spill or slow leak before it becomes an emergency.
Thick metal or high-density polyethylene containers give the best shot at stopping leaks. Old rusty drums invite disaster. One cracked drum in a corner corner ruins more product than most people realize, and cleanup can empty a budget fast. I remind everyone to update logs after restacking or moving containers—records make it much easier to track down problems later.
Strong labels cut through the confusion on busy shelves. There’s nothing more frustrating than grabbing a container only to squint at smeared ink. Permanent markers and industrial-grade stickers take the temperature swings and spills better than basic store-bought options.
PGEA is flammable, so open flames have no place nearby. Forklift traffic, static sparks, or even forgotten cell phone chargers bring risk. Chemical safety works best with a little paranoia—a mop and bucket won’t cut it for solvent spills. Spill containment pallets, sand buckets, and absorbent pads should never gather dust in the corner. Sometimes, fire-resistant storage cabinets provide the only real buffer for small cans.
Those yellow safety signs serve a real purpose too. Training new staff each season makes more difference than all the warning stickers combined. Dragging through a routine spill drill once each quarter builds muscle memory and confidence when alarms blare.
Thin latex gloves melt almost instantly with these solvents, so nitrile or neoprene gloves take priority. Lab coats or coveralls cut down on skin exposure. Goggles with foam seal edges block off splashes better than cheap safety glasses. Facility managers who walk the floor actually wearing this protective gear set a tone for everyone else—it says time and money go to keeping people safe, not just ticking off compliance boxes.
Any leftover product or old samples go out with certified waste haulers, not tossed into the trash. In just a few years working in this field, I've seen shops lose thousands from a single mislabeled drum showing up at a landfill. Transport companies carry documentation, and they don't just check boxes—they expect tightly closed lids, full manifests, and honest hazard reports every step of the way.
No matter how tempting shortcuts might look, safe handling and storage keep everyone working and production moving. Instead of chasing paperwork after an accident, the whole team builds trust—and that trust does more than any safety manual ever could.
Propylene glycol ethyl ether acetate doesn’t show up in household cabinets, but you’ll find it in labs and industrial settings where folks mix coatings, inks, and cleaning fluids. Blending this solvent with other chemicals sounds easy. Just pour, stir, and move on. In reality, it can cause plenty of headaches if treated casually.
Mistakes in mixing can spell trouble for workers, equipment, and anyone in range of a bad spill. Chemical compatibility doesn’t always follow gut instinct. For example, tossing this liquid into a vat with strong acids or bases can set off unpredictable reactions—think of bad fumes or unwanted heat. The danger isn’t just about ruined products. It’s about people dealing with chemical burns, breathing in toxins, or fumbling through cleanup with no clue what just happened.
Anyone who has worked shifting barrels in a paint factory knows the smallest errors can cost the most. Once, a colleague rushed and pumped a drum of this solvent straight into a line feeding alkyd resins. The resin thickened and gelled, turning a day’s progress into two days of rework. Nobody likes throwing out hundreds of liters of ruined material. That memory sticks. It drives home the point: never skip those chemical compatibility charts or advice from suppliers.
Plant managers keep Material Safety Data Sheets nearby for a reason. Even if someone insists “we’ve always done it this way,” chemical supplies, especially solvents like this one, can swap formulations. Industries tweak recipes and change purity grades, so what mixed safely last year can cause headaches after a subtle change.
Propylene glycol ethyl ether acetate dissolves both water-based and oil-based substances. That makes it useful, but not universally friendly. Strong oxidizers can turn it into a fire risk. Reacting it with strong acids or bases can change its chemical structure, sometimes generating gases or causing it to separate. Handling unfamiliar blends puts workplaces at risk for fires, explosions, and toxic exposure.
Over the years, studies reported by the National Institute for Occupational Safety and Health show this solvent irritates eyes and skin and can cause breathing problems at high vapor concentrations. Easy mixing doesn’t excuse careless work. Any chemical that promises to dissolve almost anything carries the hidden message: check compatibility charts every time.
No one walks into a workshop hoping for an emergency shower. Avoiding those disasters starts with training. Each team member should learn basic chemical categories and the key signals for unsafe combinations. It helps to keep real examples posted near mixing stations—a splashy sign showing what happened during that infamous resin gel-up gets remembered.
On the supply side, companies should push for better labels and plain-English hazard summaries on every drum. Not every worker can decode long chemical names after an eight-hour shift, so clearer labels and reminders mean fewer mistakes. Automated systems that scan and verify what’s being mixed can strip out a layer of human error. Small investment, big payoff.
Mixing chemicals like propylene glycol ethyl ether acetate isn’t a guessing game. It rewards respect, care, and a habit of double-checking every label and compatibility chart. No shortcut beats a safe, well-informed approach, especially in a world where one mistake can clear a whole building for hours.
Talk to anyone who’s worked around coatings or solvents, and they’ll probably know Propylene Glycol Ethyl Ether Acetate—often called PGEA. You find it in paints, inks, and cleaning agents. Its appeal shows up pretty clearly when people need a solvent that offers strong performance but doesn’t have the harsh bite or aggressive evaporation rate that some older choices delivered.
Spend a bit of time with PGEA, and you notice it has a faint, sweet odor—nothing too overpowering. This liquid remains clear and colorless in the bottle. Pour it out, and it flows much like water but with a slightly slicker feel. At room temperature, it refuses to solidify, sporting a boiling point above 170°C. That means you aren’t watching your solvent vanish within minutes, which can make a real difference on a summer day in an open shop.
Another thing people appreciate is its slow evaporation. A high boiling point goes hand in hand with less volatility. In industrial settings, that matters. Workers breathe in fewer vapors, and coatings have more time to settle and spread evenly. PGEA handles both polar and non-polar substances, so it breaks down and blends with a wide range of resins or additives. This makes it useful for more than just one kind of product.
PGEA brings stability to the table. It resists strong acids and bases, and it won’t react unless you push it hard with heat or catalysts. Mixed carefully, it stays out of trouble and doesn’t release any wild byproducts during normal use. This helps reduce headaches for lab safety officers and anyone concerned about unexpected reactions.
Some folks worry about toxic solvents. With PGEA, the risk drops. According to EPA reports, this solvent shows lower toxicity compared to things like ethylene glycol ethers, which have a history of health risks. Handle it with gloves and goggles as with any chemical, but you don’t see the same red-flag warnings from international agencies.
Factories often deal with solvents evaporating too quickly or bringing unpleasant odors that stick around. PGEA sidesteps both issues. It gives workers enough time for application without forcing them to rush, and it won’t make a whole building stink for hours. Still, letting it build up in a closed space isn’t wise, as it is flammable. Anyone using it should keep proper ventilation running and avoid sparks or open flames.
Disposal presents another concern, as with nearly all organic solvents. Here, regular collection for professional chemical waste services remains the only responsible route. Dumping it down the drain only causes bigger problems, both for pipes and downstream treatment plants.
It can be tempting to overlook the nuances of a solvent if all you want is something to thin paint or dissolve a resin. In my own experience, though, a little knowledge helps save time, money, and frustration. I’ve seen PGEA extend the usable life of coatings, cut down on equipment cleanup, and lower the headache factor for crews working indoors. Its physical and chemical behavior isn’t just textbook knowledge—it shapes the outcome of any project it touches.
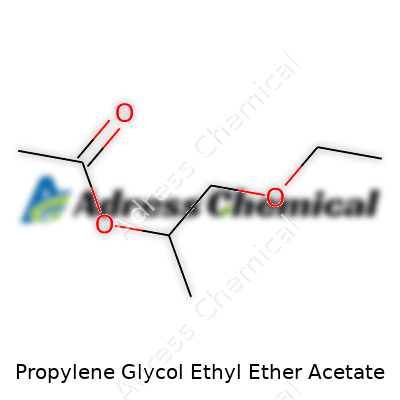
| Names | |
| Preferred IUPAC name | 1-Ethoxypropan-2-yl acetate |
| Other names |
Ethoxypropanol acetate Propylene glycol monoethyl ether acetate 1-Ethoxy-2-propyl acetate PGEEA PGEA |
| Pronunciation | /ˈproʊ.pɪˌliːn ˈɡlaɪ.kɒl ˈɛθ.ɪl ˈiː.θər əˈsiː.teɪt/ |
| Identifiers | |
| CAS Number | 2370-74-1 |
| 3D model (JSmol) | `JSmol.loadInline('3dmodel", "data", "mol", "Act as Propylene Glycol Ethyl Ether Acetate: \n\ -OCCOC(C)COC(=O)C\n\ ')` |
| Beilstein Reference | 1523674 |
| ChEBI | CHEBI:88073 |
| ChEMBL | CHEMBL1687789 |
| ChemSpider | 107782 |
| DrugBank | DB14177 |
| ECHA InfoCard | 100.047.848 |
| EC Number | 205-500-4 |
| Gmelin Reference | 102369 |
| KEGG | C19696 |
| MeSH | D065440 |
| PubChem CID | 12055 |
| RTECS number | KK8225000 |
| UNII | RD88A16Y6D |
| UN number | UN3271 |
| Properties | |
| Chemical formula | C9H18O3 |
| Molar mass | 188.24 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild, ester-like |
| Density | 0.963 g/cm3 at 25°C |
| Solubility in water | miscible |
| log P | 0.43 |
| Vapor pressure | 0.35 mmHg (20 °C) |
| Acidity (pKa) | 13.16 |
| Basicity (pKb) | > 7.7 |
| Magnetic susceptibility (χ) | -7.68×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.406 |
| Viscosity | 0.95 mPa·s |
| Dipole moment | 3.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 383.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –576.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | ΔcH⦵298 = –3554 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | Flame, Exclamation mark |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 66°C |
| Autoignition temperature | 224 °C |
| Explosive limits | Upper: 8.5% ; Lower: 0.7% |
| Lethal dose or concentration | LD50 oral rat 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,348 mg/kg (rat, oral) |
| NIOSH | R268 |
| PEL (Permissible) | Not Established |
| Related compounds | |
| Related compounds |
Propylene glycol methyl ether acetate Ethylene glycol ethyl ether acetate Propylene glycol methyl ether Dipropylene glycol methyl ether acetate Ethylene glycol butyl ether acetate |