Decades ago, the chemical industry hunted for safer and more effective solvents, especially ones suitable for paints, coatings, and cleaning solutions. Propylene glycol diacetate came into the picture as a response to tighter regulations around volatile organic compounds and worker safety. Early chemists understood that blending the flexibility of a glycol ether with improved solvency and lower odor would attract attention from formulators. Over the years, continued pressure mounted to limit workplace exposure to hazardous chemicals. Facing these demands, manufacturers placed extra effort on refining techniques for purifying glycol derivatives and minimizing impurities so that chemical standards aligned with stricter global guidelines. Production expanded quickly across North America, Europe, and then into Asia as large-scale resin and printing industries saw clear benefits compared with harsher, legacy solvents.
Propylene glycol diacetate, often called PGDA or 1,2-Propylene glycol diacetate, turns up in safety data sheets under chemical registration numbers including CAS 623-84-7. Sometimes it’s labeled as propanediol diacetate or referred to by various trade names depending on the manufacturer. The liquid’s popularity comes from its gentle odor, solid solvency, and moderate evaporation rate, opening doors to markets needing substitutes for high-toxicity ethers or esters. Common in paint strippers, degreasers, latex coatings, and printing inks, its versatility appeals to formulators looking for reliable alternatives without sacrificing performance.
PGDA stands out as a clear to slightly yellowish liquid. It typically clocks in with a boiling point between 230°C and 245°C, so it holds up under higher temperatures common in industrial paint baking. The density hovers around 1.08 g/cm³ at room temperature. Viscosity stays low enough to help blends flow, which helps when working with thick emulsions or specialty adhesives. This solvent dissolves many common resins, nitrocellulose, and dyes, yet dissolves in water only with some effort due to those ester groups. Many laboratories and quality control folks check refractive index and flash point—PGDA’s values fall into worker-friendly ranges, meaning less worry about fire risks compared to fast-evaporating, low-boiling ethers.
Strong technical control matters here. Commercial grades are generally sold with a purity of over 99%, tested by gas chromatography and acid number. Labels and MSDS sheets point out its specific gravity, moisture content, acidity, and color index. China and the EU both require details about handling precautions, recommended personal protective equipment, and spill procedures right on shipment paperwork. Labels highlight the CAS number, batch code, and clear storage guidelines. Companies paying attention to regulatory changes keep spec sheets up to date as health standards evolve—especially if PGDA is bound for export or sensitive applications where trace impurities cause trouble down the line.
Manufacturers rely on a simple but precise esterification reaction, pairing propylene glycol with excess acetic acid under acid catalysis—typically with a strong mineral acid or a solid resin. Temperature and reaction time are juggled to balance full conversion with minimal byproduct formation, while vacuum stripping removes residual acetic acid and water after the reaction. Experienced operators know that small shifts in catalyst ratio or temperature give big swings in product purity. Final distillation and filtration ensure a clean, nearly colorless product ready for demanding customers, especially those running continuous processes with little room for unexpected solvent impurities.
PGDA’s two ester groups lend themselves to predictable chemistry. Under alkaline hydrolysis, those acetates flip back to propylene glycol and acetic acid. Aqueous solutions at elevated pH tend to break ester bonds faster, so storage in metal containers is usually avoided unless well-lined against corrosion. Enhancing performance for niche markets—like waterborne coatings—can mean tweaking blend partners or reacting PGDA with other glycol esters to change volatility and solvency. Many R&D labs test its compatibility with reactive resins, especially when chasing faster dry times or improved pigment wetting.
Unlike harsh acetates or aromatic solvents, PGDA avoids strong toxicity risks under normal conditions, yet it’s not entirely harmless. Vapors can irritate eyes and airways in confined spaces. Prolonged skin exposure leaves workers open to mild dermatitis. Most plants use basic engineering controls—extraction fans, gloves, goggles—rather than high-grade respirators, since atmospheric concentration rarely tops legal limits during standard use. Spills clean up easily thanks to low volatility and modest water solubility. Disposal has grown more regulated with environmental rules tightening around spent solvents, so wastewater pre-treatment gets checked weekly and drums picked up by certified handlers. There's less disaster risk, but nobody wants to see fines over missed collection schedules or improper labeling.
PGDA finds a home where balanced solvency is the name of the game. Decorative and industrial paints benefit from its middle-range evaporation rate. Flexographic and gravure inks use it to rotate between quick transfer and steady printability. Electronics flux removal, cleaning machinery, and even textile printing draw from its strengths. Water-reducible stains and coatings happily accept a splash for flow adjustment without sacrificing film clarity. Agricultural adjuvants get a lift in tank-mixing by solubilizing stubborn additives. Some labs have explored using it in specialty adhesives because of its resistance to water whitening—a big plus for manufacturers tired of bubbling or haze during humid storage.
With tougher emissions rules and less tolerance for worker exposure to traditional aromatics, R&D teams took a hard look at glycol diacetates for safer workplace chemistry. They ran performance trials against legacy solvents—testing dry times, gloss, film resistance. Many reported that PGDA stood out in low-odor coatings and indoor products aimed at sensitive environments like hospitals or schools. Past studies examined how blending with slower or faster solvents altered application profiles, hoping to fine-tune everything from brushability to block resistance. Recent patents seek biodegradable glycol derivatives, less persistence in aquatic environments, and even easier downstream recycling or water treatment. Ongoing collaborations between chemical producers and universities keep feeding the innovation pipeline, especially as industry pushes for circular economy approaches.
Safety data indicates that acute oral, dermal, and inhalation toxicity ranks much lower for PGDA than for many comparable esters. Lab studies in rodents showed high LD50 values, signaling low immediate risk from accidental contact. Chronic exposure tests looked for organ or reproductive effects and found little to worry about within occupational settings. Environmental fate checks show moderate biodegradability, so routine use won’t leave trails of persistent chemicals in water or soil. Regulatory agencies still recommend limiting exposure for pregnant workers and monitoring indoor air through periodic workplace sampling, just to keep long-term risk in check as more workplaces switch their go-to solvents.
Demand for PGDA will probably climb in step with paint and coating reformulation trends, especially as stricter VOC caps arrive on both sides of the Atlantic and Pacific. Advances in enzyme-catalyzed esterification and green chemistry offer reasons to expect even purer, lower-carbon production routes. More companies explore ways to recycle leftover solvents or recover PGDA from waste streams, harnessing closed-loop systems to meet sustainability pledges. As water-based technologies edge out solvent-heavy processes, chemists keep searching for glycol esters that solve technical headaches without environmental tradeoffs. Expect continued partnership between manufacturers, customers, and watchdog agencies to hold product stewardship front and center, shaping the next chapter for propylene glycol diacetate.
Propylene glycol diacetate sounds technical, maybe even a bit intimidating. In reality, this compound shows up in more everyday places than folks expect. Take a walk through any hardware store, and the scent of fresh paint often drifts through the air—that’s where this chemical quietly does its job.
This ingredient steps up mainly in paints, coatings, and inks. Propylene glycol diacetate makes it easier to spread paint evenly across a surface, and it stops the finish from drying up too quickly. Many companies also turn to it for printing inks, helping the colors stay vibrant and smooth, especially for those glossy magazines and packaging labels.
It’s not only limited to making things look good. Manufacturers found that this ingredient plays a big part in helping coatings last longer, even under tough weather. Paint flaking off your fence or ink fading from packaging labels leaves a bad impression, and this solvent helps prevent that headache.
Some paint products release strong fumes, which not only annoy people but can cause health issues. Propylene glycol diacetate manages to cut down on the harsh smell and the risk, compared to several other solvents used in the past. During my own home renovation, the headache and burning eyes from certain paints and cleaners stuck with me. Knowing there’s a safer alternative matters.
Regulations also keep tightening on what companies are allowed to put into their products. Solvents like xylene or toluene faced bigger restrictions over time thanks to health concerns. By shifting toward ingredients that evaporate more gently and leave behind fewer toxic residues, paint and ink makers can stay ahead of new rules while protecting workers and customers.
The United States Environmental Protection Agency and European regulators both keep a close eye on ingredients like this. Compared to older, harsher solvents, propylene glycol diacetate sits lower on the totem pole for potential danger. It breaks down more easily in the environment, which cuts down on long-term pollution.
The paint and coatings industry is massive—worth well over $160 billion globally as of 2024. People want products that roll on smooth, keep color locked in, and don’t come with a scary warning label. Manufacturers found that this compound helps answer all those demands without forcing up costs or adding unnecessary risks.
Switching every formula over to something “safer” or cleaner doesn’t always work right away. There’s give and take, especially in how a product handles during real-world use. For example, too much focus on a “green” label can reduce durability or increase price.
What has made a difference is growing demand from consumers—people reading labels and pushing for transparency. After years of DIY projects and watching friends react to strong chemical smells, I’d rather see more research into how these solvents blend safety with performance. There’s still room to improve not just the formula, but the whole experience, so indoor projects don’t feel like a gamble with your health.
If paints and inks can rely on ingredients like propylene glycol diacetate, companies can worry less about regulatory drama, and families can enjoy safer indoor air. Still, it’s important not to take these changes at face value. Reading up, asking questions, even reaching out to makers for detailed information, helps everyone make smarter decisions about what gets brushed onto walls or printed onto packaging.
Most folks want to know what’s inside the things they eat and put on their skin. Food and cosmetics travel through our lives every single day, and the list of chemical names on a package often brings more questions than answers. Propylene Glycol Diacetate (PGDA) pops up in ingredient lists for both snacks and skin creams. Whether you’re a label-reader at the grocery store or someone chasing solutions for skin comfort, the safety of this ingredient deserves a closer look.
PGDA isn’t some rare chemical that only shows up in a lab. It comes from propylene glycol—a substance often used as a solvent in foods and personal care items—reacted with acetic acid. The result? A clear liquid with a mild odor, and its main job is to help dissolve flavors, fragrances, and actives so the end-product performs as promised, stays smooth, and feels pleasant.
In food products, PGDA usually acts as a carrier or solvent for flavors. Regulators like the FDA in the U.S. and EFSA in Europe keep a close eye on additives, requiring a decent stack of safety data before declaring anything safe for food contact. Research finds no proof that PGDA, in the small amounts approved for food use, causes harm. It breaks down inside the body into harmless substances, similar to what you’d find in a salad dressing mixed with a shot of whiskey—acetic acid and propylene glycol, both well known and studied. Most governments set clear limits on how much can appear in a finished snack, which keeps the intake well below amounts linked to unwanted effects.
Cosmetic chemists reach for PGDA because it dissolves scents and preserves the feeling of a silky formula. Its track record in leave-on and rinse-off lotions leans positive. Most studies don’t find problems like stinging or allergic rashes in day-to-day use, except for a tiny fraction of folks who react to nearly any outside chemical. Dermatologists say this stuff lands in the same camp as propylene glycol, which means it won’t make most people itch or break out, but everybody’s skin has its own opinion. For big groups of people, including children and folks with sensitive skin, regulators still say it checks out as safe at approved levels.
Chemical safety isn’t set in stone. New discoveries roll out every year, and scientific opinion changes when the evidence shifts. For PGDA, decades of lab testing and real-world use seem to support its spot in food and skin products. Of course, problems can hide in long-term exposure that doesn’t get caught in short-term studies. Most experts suggest keeping food additives simple where possible and supporting new research that checks for risks, especially for kids or people with immune conditions.
Shoppers drive change with their wallets. Companies step up transparency when customers start asking questions. More detailed labeling, access to up-to-date safety data, and tighter limits for food and personal care items could all add trust. For anyone worried about chemical ingredients, it’s always worth reaching out to the brand or picking certified organic options, which avoid most synthetic solvents altogether. Knowledge can travel a long way, and pushing for honest talk from both companies and regulators makes the space safer for everyone in the long run.
Some folks hear the name “Propylene Glycol Diacetate” and their eyes glaze over. That’s understandable. This isn’t a household staple, and you won’t spot a bottle sitting next to your flour or sugar. Still, this clear, colorless liquid holds a spot in a surprising number of products. You might notice its faint sweet, fruity smell if you encounter the pure stuff, but it’s more likely mixed into something else by the time it reaches your hands.
Its basic fingerprint: a moderate boiling point around 190°C and a relatively low viscosity. It behaves as a solvent—meaning it’s great at dissolving or thinning things that don’t budge for water alone. It’s also less dense than water, letting it float when the two meet. One thing that stands out: Propylene Glycol Diacetate doesn’t easily catch fire but won’t win any gold medals for resistance, either. It lands in a middle ground—not too flammable, not entirely safe from an open flame, either.
I ran into this chemical while working on a warehouse floor, shipping shipments of paint and cleaning mixtures. Those products need more than color and scent—they lean on solvents like this one to spread smoothly and perform. From personal care sprays to degreasers, Propylene Glycol Diacetate quietly boosts performance while staying largely invisible to end users.
Industrial pros like it because it can dissolve oils, resins, and some plastics where others give up. Its odor isn’t overpowering, so manufacturers choose it for applications that go directly on skin or in the air. Plus, it doesn’t linger in your lungs like some harsher solvents. That doesn’t mean you should breathe it in on purpose—it’s still best handled with basic safety gear like gloves and goggles.
Each chemical comes with upsides and downsides. Propylene Glycol Diacetate stands up to gradual breakdown in moist air. Over time, water and acid can split it apart into smaller acids and alcohols. In a tightly closed bottle, it’ll hang together for ages. If you spill it, it won’t evaporate in minutes like acetone or alcohol. That can be a good thing for slow-drying paints or coatings but makes cleanup stickier if there’s a mess.
Environmental impact deserves mention. Unlike some old-school solvents, this one breaks down more easily outdoors. It doesn’t build up in the food chain, and if you handle waste correctly, you avoid most environmental headaches tied to less friendly chemicals. Still, flushing gallons down a drain spells trouble. Proper disposal keeps it out of streams and water tables.
No matter the material, responsibility lands on everyone handling chemicals. Training, good ventilation, and simple protective gear go a long way. In the lab and on the warehouse dock, clear labeling and MSDS sheets back up common sense. Most workplace incidents stem from skipping these basics.
There’s a real push in industry to swap out rougher solvents for safer ones. Propylene Glycol Diacetate gets the nod more often now, not just for performance—it leaves a lighter mark on people and planet. Companies still need to keep an eye out for safer alternatives, but right now, this one’s a decent compromise between effectiveness and safety for people who work with it and for the world outside.
Propylene Glycol Diacetate tends to show up in places many wouldn’t expect, from paints to flavors. It isn’t something people chat about at dinner tables, yet those handling it see the importance up close. Over time, I’ve seen what happens when storage gets sloppy — small mistakes can spark big headaches.
Keeping this stuff upright means thinking about more than just a corner of the warehouse. I once worked in a spot that tried to stuff as many barrels as they could on a single rack. Didn’t end well. Leaks crept out, vapors grabbed noses before anyone realized, and cleanup wasted a full afternoon. From then on, I paid closer attention to ventilation and a cool, dry spot with steady air flow. Sealed containers, nothing left to chance. People sometimes downplay the flammable nature, but it takes one spark in a crowded space to get the whole team talking safety real quick.
Anyone working with Propylene Glycol Diacetate shouldn’t rely only on gloves and a lab coat. I remember a friend skipping eye protection because “it looked safe.” A tiny splash proved her wrong. Goggles, gloves, and long sleeves aren’t suggestions — they’re what keep lunch breaks at lunch instead of urgent care. Protective gear makes a difference and good training shows everyone why.
Every safety sheet rattles off temperature and humidity ranges for a reason. Around 25°C tends to keep things stable; let it swing higher, and the smell becomes bold, letting you know you missed something. I once checked on a batch left near sunny windows. Strong odor jumped out and the product didn’t work right after that. It’s not just about product loss; the wrong storage area pushes costs up and trust down.
No matter how careful you are, barrels leak, jugs crack, or caps don’t seal. I’ve scrambled to contain a spill with nothing nearby but old rags. Lesson learned: keep real spill kits close by — absorbents, neutralizing powders, plenty of disposable bags. Quick cleanup matters, but safe disposal should follow. Drains aren’t magic erasers.
Mistakes aren’t rare, but repeating one points to the wrong habits. Leaving containers open, mixing storage with unrelated chemicals, or skipping daily checks cause more problems than they save time. Regular inspections, honest record-keeping, and easy-to-see hazard labels keep surprises away.
Not every warehouse sits in a perfect spot or comes with the latest features. Still, companies can add fans, upgrade monitoring tools, or just set aside space away from high-traffic or high-temperature areas. I’ve seen small outfits add simple warning signs or use colored floor tape to mark safe zones. Training isn’t just for newcomers; refreshers save stock and prevent injuries.
In the end, safe storage and handling aren’t just checkboxes. They’re grounded in practical steps, hard-earned lessons, and teamwork. People buy time and peace of mind by sticking to the basics. It only takes one missed step for problems to multiply, but each careful action pays off over and over — in safety, in quality, and in the stories we don’t have to tell.
Propylene glycol diacetate doesn’t show up in catchy ads or loud TV commercials, but plenty of industries blend it into products. Painting a living room? Peeling a fruit? Spraying a perfume? You might end up close to this clear liquid. It shows up in solvents, fragrances, and even some food flavorings. So, it deserves more than a passing glance, especially if there’s any risk to health.
Whenever I help a neighbor with some paintwork, I notice faint chemical scents drifting through the air. In those moments, stories about headaches or coughing feel less like warnings and more like real possibilities. Propylene glycol diacetate can irritate the eyes, nose, and throat, especially if the exposure happens indoors or for long stretches. A strong smell or tickle at the back of the throat usually tips people off. I’ve seen a few coworkers rub their eyes, looking for a way outside after breathing it in too long during a renovation job.
We talk about irritation, but it doesn’t end there. Some people run into dizziness or nausea if the fumes build up. Painting in small rooms often traps vapors, and I’ve heard stories of headaches lingering for hours afterward. Accidental contact with the skin can lead to a rash — not every time, but for people with sensitive skin, it matters. Ingesting the liquid isn’t likely unless there’s a bizarre accident or a workplace spill, but if it happens, stomach pain or vomiting might follow.
Day-to-day life rarely brings danger from propylene glycol diacetate for most folks. The real problems show up for people mixing and using it every day at work — factory workers, house painters, or folks blending flavors and fragrances. Hands-on exposure, day in and day out, adds up. Some studies link high exposure levels to problems like depressed breathing rates or effects on the nervous system in animals. Research in humans remains thin, but nobody wants to gamble with long-term health just to avoid wearing gloves or running a fan. Precaution beats regrets.
Some folks believe any approved chemical is harmless in tiny doses. It’s possible to treat the sniffle or sore eye from the occasional project as something minor, but there’s more to it. Long-term, low-level exposure builds up. Sensitive groups—children, pregnant people, those with asthma—may feel effects quicker. Regulations cap the amount allowed in food and air for a reason. Safety rules make sense, even if the risk looks small on paper.
A few steps make a big difference. Open windows wide during painting, cleaning, or spraying fragrances. Protective gloves and goggles don’t cost much. Bigger workplaces need proper air systems to sweep out fumes. I always check for warning labels and never skimp on the safety gear, even if the job seems quick.
The more we learn about chemicals like propylene glycol diacetate, the clearer it becomes that health wins when we don’t take shortcuts. Easy precautions can turn potential hazards into simple workday routines—saving headaches, both literally and otherwise.
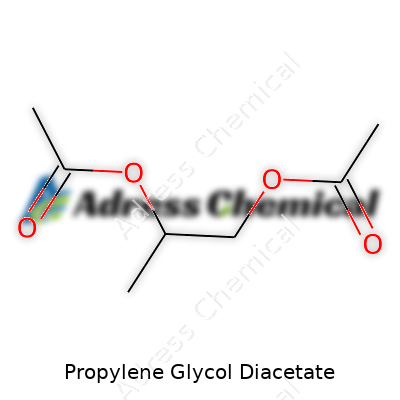
| Names | |
| Preferred IUPAC name | 4-hydroxy-4-oxobutanoic acid 1,3-diacetate |
| Other names |
1,2-Propanediol diacetate PGDA Diacetin Diacetoxypropane |
| Pronunciation | /ˈproʊ.pəˌliːn ˈɡlaɪ.kɒl ˌdaɪ.əˈseɪt/ |
| Identifiers | |
| CAS Number | 623-84-7 |
| Beilstein Reference | 773158 |
| ChEBI | CHEBI:86455 |
| ChEMBL | CHEMBL58208 |
| ChemSpider | 19978548 |
| DrugBank | DB14096 |
| ECHA InfoCard | ECHA InfoCard: 03-2119753879-15-0000 |
| EC Number | 434-250-7 |
| Gmelin Reference | 8704 |
| KEGG | C19600 |
| MeSH | D011377 |
| PubChem CID | 8219 |
| RTECS number | UF3325000 |
| UNII | L9H7M72IT8 |
| UN number | 3272 |
| CompTox Dashboard (EPA) | DTXSID5020656 |
| Properties | |
| Chemical formula | C7H12O4 |
| Molar mass | 188.22 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild, fruity |
| Density | 1.06 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.08 |
| Vapor pressure | 0.07 mmHg (20°C) |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 15.24 |
| Magnetic susceptibility (χ) | -9.71×10^-6 |
| Refractive index (nD) | 1.415 |
| Viscosity | 2.2 mPa·s (25 °C) |
| Dipole moment | 4.2 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 379.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -933.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2628 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D01AE25 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 108°C |
| Autoignition temperature | 210 °C |
| Explosive limits | Explosive limits: 0.9–9.6% |
| Lethal dose or concentration | LD50 oral rat 5660 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 5,400 mg/kg |
| NIOSH | 'AS3150000' |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
Propylene glycol Diacetin Ethylene glycol diacetate Propylene glycol monoacetate |