Decades ago, chemists needed solvents that could help dissolve grease, oils, and pigments without creating hazards or toxic fumes. Propylene Glycol Butyl Ether (PGBE) came out of this push for practical, safer solvent options in the 1950s and 60s. It gained traction because it outperformed older glycol ethers, offered lower toxicity than many alternatives, and worked across a wider temperature range. Industry started turning to PGBE as manufacturing ramped up, especially for cleaning and coatings, after companies learned it cut down on both pollution and worker complaints. Seeing the name keep popping up in cleaning product ingredient lists for years shows just how much it changed the landscape for manufacturers and maintenance crews alike.
Manufacturers make PGBE as a clear, nearly odorless liquid. You’ll recognize it by its mild, sweet scent reminiscent of other glycols, though less intense. Factories sell it in drums marked with UN numbers and product IDs, sometimes as “propylene glycol mono-n-butyl ether,” “PNB,” “Dowanol PnB,” or “Glycol Ether PnB.” Producers keep it in steel or high-density polyethylene containers, tightly sealed to avoid excess moisture. You’ll find it distributed globally, with multinational chemical companies pushing it to paint shops, cleaning supply depots, and automotive centers by the ton.
PGBE sits at about 162°C boiling point and doesn’t freeze unless temperatures fall below -80°C. It carries a specific gravity close to 0.88 at 20°C, making it lighter than water. Soluble in both water and a long list of organic solvents, PGBE can break up stubborn dirt and stains. It evaporates slowly, letting cleaners linger and do a thorough job. Properties like flash point above 68°C allow it to get used in settings where sparks fly or temperatures run high, reducing fire risk. Quality control labs check for consistency in color, refractive index, and water content—details listed on technical sheets accompanying every shipment.
Regulatory agencies ask chemical plants to label each drum with hazard pictograms, CAS number (5131-66-8), and warnings about skin or eye contact. Some countries assign safety data sheet numbers and emergency phone references. The shipping paperwork details concentrations, recommended storage conditions, and first-aid steps. Workers read product labels showing percentage purity, recommended dilution rates for cleaning, and international transport codes. These details don’t sit just for show—occupational safety teams rely on them daily to check compliance and prepare the workforce.
Factories synthesize PGBE in reactors by combining propylene oxide with n-butanol, relying on catalysts under carefully controlled pressure and temperature. Operators monitor each batch for moisture, byproduct formation, and purity—skilled hands know a small slip can generate foam, waste, or off-odor. The last steps involve distillation to remove unreacted alcohols and acids, testing the final cut for clarity, and pumping it to storage. Many workers tell stories of leaks or over-heating back in the control room, driving home how finely tuned these processes are.
PGBE’s molecular structure means it tolerates acids, bases, and light oxidizers, yet reacts with strong agents like sodium metal or concentrated nitric acid. Chemists sometimes tweak the butyl group to make new glycol ethers or react the ether with acrylates to build UV-curable coatings. Projects in adhesives labs often pull in PGBE as a “reactive diluent,” letting materials flow better without making adhesive joints brittle. Its balance of hydrophilic and lipophilic tendencies helps labs modify surface tension and compatibility for everything from inks to surfactants.
PGBE rarely gets sold under a single label. The chemical catalogs list it as “Propylene Glycol n-Butyl Ether,” “1-Butoxy-2-propanol,” “Dowanol PnB,” and “PnB Glycol Ether.” Some local suppliers twist the name for marketing or registry purposes, like “Arcosolv PnB” or “Glysol PnB.” Tech-savvy buyers double-check product codes and batch data to avoid confusion, since substitutes carry different tox or flammability risks. Any mistake here makes for headaches down the line—mixing up with similar-sounding glycols can spoil an entire blend, especially for paint shops.
Though it’s safer than many harsh solvents, PGBE still calls for careful handling. Anyone working with raw product suits up with gloves and goggles, keeps workrooms ventilated, and posts safety signage near mixing stations. Spill kits stocked with absorbents stay on hand wherever it’s stored in bulk. Companies train custodians and factory floor staff to recognize symptoms like headaches or eye irritation after exposure, sending them to wash up and report for health checks if needed. Environmental monitors test air for vapor concentrations, and any accidental discharge gets logged for follow-up. Compliance officers walk the site every week, checking storage drums for corrosion or overfill.
What keeps PGBE in high demand is how it straddles multiple industries at once. Paint labs use it as a coalescing agent—boosting film strength in water-based products without raising solvent emissions. Floor strippers and degreasers lean on its solvency power for chewing up waxes and oil films. Printing and ink plants like how it helps dyes wet out paper, stopping colors from beading up or streaking. It pops up in agricultural chemicals, where it helps disperse active ingredients more evenly. Car care lines, including polishes and windshield cleaners, blend it in for residue-free cleaning. Factories keep PGBE on hand because each production line—paint, coatings, adhesives, cleaning, and more—leans on its unique blend of solvency and safety.
University and corporate labs keep pushing for greener, safer solvents, but PGBE remains a reference point. Chemists test alternative chain lengths and ether variants, trying to mimic PGBE’s balance of vapor pressure and toxicity. Ongoing R&D looks at bio-based glycol ethers and improved recycling methods for spent solvents. Field trials with enzyme-powered detergents often compare performance side by side with PGBE formulations. Tech conferences stack panels with real-world users who share both breakthroughs and stumbles, calling for input on how next-generation solvents stack up in shop-floor conditions.
Toxicologists track exposure outcomes over years, asking how skin absorption and inhalation doses land in people working with PGBE every day. Reports show that short-term contact brings low irritation, but repeated high exposure can cause headache, narcosis, or liver stress—much lower risks than older solvents, but still not ignorable. Animal models and workplace studies inform new regulations, squeezing down exposure limits room by room. Worker advocacy groups lobby for better air testing and medical monitoring in older plants, while companies roll out air scrubbers and automation to cut down manual contact.
Looking a few years ahead, the push for green chemistry may reshape the story for solvents, but PGBE’s long-standing reliability and versatility set a high bar. Unless safer, less expensive options show up soon, paint makers, janitorial businesses, and chemical processors will keep counting on it for the jobs that need real solvency muscle. Automation, better ventilation, and targeted substitutes in consumer products could reduce worker contact and environmental load. The chemistry classroom is already seeing new generations learn from the balance PGBE struck between performance and safety—a conversation that will keep evolving as innovation rolls forward.
Pop open a bottle of your usual bathroom cleaner and there’s a good chance you’ll spot propylene glycol butyl ether quietly listed among the ingredients. It rolls off the tongue awkwardly, but its work-horse resume makes life a bit easier at home and across industries. This clear, nearly odorless liquid falls under the bracket of solvents. In real life, folks rely on it for its knack at dissolving greasy, oily, or waxy messes. Homes, hospitals, restaurants — all keep battling stains, dirt, and things left behind on surfaces. Manufacturers pack this solvent into spray bottles precisely because it can cut through the sticky film that water alone barely budges.
Step into any paint shop, and most tins lined up on shelves owe a debt to this solvent. Without it, latex paints dry sooner than you want, making it tough to achieve a clean finish. Adding propylene glycol butyl ether keeps that paint wet just long enough for smooth strokes and proper blending. The same holds for coatings on machinery or vehicles. This solvent helps the pigment flow better — meaning fewer brush marks and much neater lines. If you’re refinishing furniture or touching up the backyard fence, chances are you’ve brushed on a layer lifted by this clear liquid.
Factories, print shops, and vehicle garages face grease on a different scale. Here, crews need something that melts away grime without eating into metal or sensitive plastics. Propylene glycol butyl ether balances strength with safety. That makes it handy in degreasers, floor cleaners, and printing ink removers. I remember a summer spent cleaning auto parts with a stubborn mix of oil and road gunk — the commercial degreasers we used didn’t leave us choking, but they got every part clean and dry in record time. It’s all about finding a cleaner that works hard without turning the job into a safety risk.
This solvent hides in places most people wouldn’t suspect. Window sprays often tap into its power to leave glass streak-free. Dry-cleaning outfits appreciate its ability to break down organic spots before clothes go through the wash. Even the printing business trusts this ingredient — enabling vibrant, smudge-resistant magazine covers and food packaging. The magic comes from its ability to dissolve and suspend a whole range of compounds — which means fewer residue problems and a crisp end result.
Some concerns follow any solvent with a chemical name nobody can pronounce. People wonder about health effects and what all these names mean for air quality indoors. Agencies like the EPA do keep a close eye and set strict guidelines on how much can be present in products. Safety always circles back to how well the workplace is ventilated or if hands and eyes are protected. I’ve learned that irritation rarely hits unless you’re soaking in it or breathing it straight — but workers staying safe still means manufacturers should always look for greener options and educate teams about what’s in their bottles. There’s always room to push for less-harmful choices, but for now, this transparent helper remains a backbone in cleaning, painting, and industrial jobs behind the scenes.
There’s a long list of chemicals found in everyday cleaning products, paints, and even a few cosmetics. Propylene Glycol Butyl Ether (PGBE) pops up often, mostly because it cleans and dissolves grease so well. Plenty of folks handle it at work, scrubbing machinery, mopping up shop floors, or thinning out industrial paints. That’s enough reason to question if it deserves a spot on the shelf at home, too.
Years of scientific digging haven’t shown PGBE as the worst offender in the cleaning world. The Environmental Protection Agency (EPA) and European Chemical Agency have both run safety checks. In small amounts, they don’t flag it for usual household use. Breathing it in, touching it, or even a small accidental taste doesn’t stack up next to big risks like ammonia or stronger solvents. Most side effects start with skin or eye irritation. Workers dealing with concentrated liquids day in and day out have the highest odds of running into problems—itchy skin, red eyes, sometimes a mild sore throat. Large spills, or using this stuff without gloves, can up the chances.
What’s less clear is how it acts over time. Nobody knows yet if slow, steady exposure might sneak up with new problems. The EPA says cancer isn’t linked to PGBE, and there’s not much proof it messes with genes or causes birth defects. That’s a relief, but long-term workers might want to take care, especially in places without much ventilation. My personal experience in auto shops showed that skipping gloves made knuckles crack and sting by the end of a shift, but covering up put that right.
People get tripped up mixing household cleaners. Few read the labels or notice warnings. Tossing a PGBE-based cleaner together with bleach or ammonia brings a chemical soup nobody wants to breathe. These mixes can fill a space with vapors that scratch the throat and burn the eyes. I’ve inhaled that mistake cleaning bathrooms in older apartments. The lesson stuck: open windows, keep chemicals apart, and step away if the fumes sting.
Relying on labels can pay off. Glove up, wear safety glasses, and don’t linger over open bottles or buckets. Folks at home mostly see PGBE in low amounts, not the strong industrial stuff. Air out rooms after a deep clean or paint job. If something splashes on the skin, wash it off right away. Habits like these work, plain and simple—much like washing hands after dealing with any cleaning product.
Factories use more PGBE, and that’s where oversight matters. Government rules help, but some worksites run lean on protections. Shortcuts tempt managers to save cash, even as better ventilation or safety gear could avoid sick days and lawsuits. OSHA sets exposure limits, but workers need bosses to listen and act before trouble shows up. Nobody deserves red eyes or rough skin just for doing their job.
Alternatives exist, often labeled “green,” but not every gentler cleaner scrubs away oil or dried paint. Sometimes elbow grease and patience win over synthetic chemicals. Consumers and companies could nudge markets by choosing safer choices and reading up on what’s inside products, instead of reaching for the strongest bottle by reflex.
If PGBE is in the cupboard, treat it with respect like any cleaning chemical. People don’t have to fear it, but paying attention to personal protection makes the difference between a clean kitchen and a rash or headache. Staying informed—about what’s being used, how much, and where—will always beat guessing blindly.
Propylene glycol butyl ether, or PGBE as it’s often called on safety data sheets, stands out in the chemical world for its versatility. I’ve come across this solvent in everything from industrial cleaners to paints at the local hardware store. Its clear, slightly sweet-scented liquid looks harmless, but the properties under the surface deserve real attention.
You’ll notice right away that PGBE comes out as a colorless liquid, thin and quick to spread if spilled. Its low viscosity helps it flow and mix with other liquids without much coaxing. Pick up a bottle and you’ll find it weighs a bit less than water—its density hangs around 0.88 g/cm³. I’ve handled it in warm and cold warehouses, and it doesn’t freeze up easily. PGBE’s freezing point hides down at −80°C, and whenever summer rolls around, you won’t see it boil until it hits about 171°C. That wide temperature window makes it useful for jobs that push the limits of most household solvents.
The scent, faintly sweet but not overpowering, comes from its relatively low vapor pressure. At room temperature, it doesn’t evaporate in a hurry, which cuts down on indoor air quality worries. But the liquid still evaporates enough that good ventilation matters, especially in bigger applications.
Chemically, PGBE belongs to the glycol ether family, so its molecules can latch onto a wide variety of substances, both water-based and oily. This dual character drives its cleaning power, pulling grease or grime from surfaces as easily as ink smears from a whiteboard. Environmental chemists often point out how solvents like this bridge the gap between polar and non-polar messes.
PGBE fights off chemical breakdown in water, and it stays stable under basic and slightly acidic conditions. Still, it’s important to remember that mixing it with oxidizers or strong acids brings unwanted reactions—those can turn a straightforward job hazardous in a flash. I’ve seen operators get careless with storage, stacking drums on top of incompatible chemicals, and no good comes of it.
The biggest challenge I’ve noticed is the balance between usefulness and safety. PGBE dissolves messes beautifully, but inhaling high concentrations or getting it on bare skin too often can irritate the body. I’ve experienced a slight stinging sensation on my hands after repeated contact, and sensitive folks can run into more trouble. Fact sheets warn against prolonged exposure, so I always reach for gloves and prefer to keep a window cracked.
There’s another side to think about: environmental and workplace risks. PGBE doesn’t break down particularly fast if it finds its way into waterways. This doesn’t make headlines, but anyone who works with large volumes knows that spills and leaks happen. Better containment and responsible disposal practices, with real buy-in from staff, help a lot. I’ve found that small changes—like proper labeling, bunded storage, and regular safety drills—make a bigger impact than endless paperwork or occasional safety speeches.
If I could give advice to anyone working around PGBE, attention to detail pays off. Using it for what it does best, keeping safety equipment close, and understanding its quirks protect both workers and the environment. Lab reports and regulations matter, but honest conversations between staff members and clear procedures work better in practice. With care and respect for its properties, PGBE can stay an effective tool rather than a problem waiting to happen.
In factories and workshops, chemicals like propylene glycol butyl ether often don’t get much attention—until someone gets careless or something goes wrong. I’ve seen more than one worker shrug off the warnings, only to end up with headaches or skin rashes after splashes or spills. This solvent helps keep paints wet and makes cleaning products more effective, but it isn’t something to treat lightly. History is packed with stories of ignored storage guidelines leading to leaky containers, ruined goods, or worse, chemical accidents. People sometimes focus on production speeds but overlook the basics that keep everyone safe.
Space in a warehouse always brings a juggling act, but some rules don’t get broken no matter how tight things get. I’ve always found it best to store materials like propylene glycol butyl ether in metal drums with tight-fitting lids. Invest in containers made out of stainless steel or HDPE; the wrong plastic can start softening or cracking. Propylene glycol butyl ether evaporates slowly, but once it has a way out, fumes will fill a room and nobody wants a fire or health scare on their hands. Keeping these drums in cool, dry spots far from open flames, heaters, or welding projects stops a lot of headaches before they start. Store it away from acids and bases—mixing those spells disaster.
Most managers ignore ventilation until somebody starts coughing or complaining, but a good airflow is a lifesaver. A well-placed vent above where you open drums pulls fumes away, making life easier for everyone. I remember one paint shop that left everything wide open. Things got stuffy, and staff started feeling off—turns out just a couple of extraction fans made all the difference.
Carrying or using this solvent means gloves, goggles, and long sleeves—no arguments. Even short-term exposure can dry out your skin or cause stinging eyes. Single-use nitrile gloves fit best for my hands. Cotton or thin poly gloves just don’t cut it and wind up getting soaked through. Chemical-resistant goggles beat regular safety glasses when you mix or pour from big containers. Spills look like no big deal at first, until you see streaks on your arms or you catch a whiff that makes your head spin.
For larger jobs, splash aprons or coveralls make sense. Boots that resist chemical spills finish the outfit. I learned early to keep a spill kit handy—absorbent pads, neutralizers, and a way to seal up leaky containers. I've seen coworkers scramble in emergencies because someone forgot to restock the kit or check expiration dates.
Talking about safety never means much until it becomes habit. New hires at my old job learned how to read Safety Data Sheets pinched beneath magnets on the fridge in the break room. It's not fancy, but people start taking it seriously when every shift leader points out the risks. Regular walk-throughs help catch leaking lids or sloppy labeling before they become toxic headaches.
I see a lot of companies ignoring these small steps, and they pay for it later—sometimes with damaged products, sometimes with sick workers, sometimes with fines. Even if rules feel like extra work, just one close call can change everyone’s mind. Safe storage and careful handling come down to common sense, good habits, and treating every drum like it could cause real harm. That keeps people healthy, and it keeps businesses out of trouble.
Propylene Glycol Butyl Ether often turns up in household cleaners, paints, inks, and coatings. It works as a solvent, a key to breaking down grease and delivering streak-free windows. So it’s not rare to find traces of it on counters, walls, or even your hands after a day’s cleaning. Exposure sneaks up from all directions, especially in people working jobs where chemicals pile up every shift. Based on my own experience, using strong cleaners in poorly ventilated rooms brings a scratchy throat and watery eyes pretty quickly. That's no accident.
A lot of people shrug off mild symptoms — a cough, itchy skin, headaches — without taking a second look at what’s behind those reactions. But ongoing contact can lead to more than momentary discomfort. According to the US Environmental Protection Agency, inhaling propylene glycol ethers can irritate the nose and throat, sometimes leading to asthma-like symptoms in sensitive groups like kids or those with lung disease. Skin contact with the liquid feels harmless at first. With repeated exposure, though, dermatitis kicks in — dry, red, flaking patches that can linger. I knew a janitor who dealt with constant hand rashes until she started wearing gloves every shift. The switch made all the difference.
Wastewater streams from factories and homes carry propylene glycol butyl ether straight into rivers and lakes. Fish and bugs downstream face the problem next. It doesn’t stick around for years like some chemicals, breaking down more quickly in air and water. But concentrations in local water systems still jar those fragile aquatic worlds, disrupting growth and reproduction for fish and invertebrates. The U.S. National Library of Medicine lists aquatic toxicity as “moderate,” and I’ve noticed reports that local fish populations dip in spots close to industrial discharge pipes. Even at low doses, chronic exposure stresses these ecosystems, making them more vulnerable to other threats, like heat waves and invasive species.
People don’t have to accept health risks as just part of the job or routine housework. Simple moves make a big difference. Gloves, good ventilation, and eye protection cut down on personal exposure. I started ventilating my house better during deep cleans and instantly noticed fewer headaches. Choosing products with less hazardous substitutes keeps residues out of waterways. More manufacturers have begun shifting toward safer alternatives. For instance, some use dipropylene glycol or even bio-based solvents.
Wastewater treatment upgrades stand as a next important hurdle. Not all plants in the U.S. can handle chemical solvents effectively. Smaller communities get hit worst, since modern upgrades cost real money. Funding innovation in treatment tech — like advanced oxidation processes — would catch solvents before they reach lakes and streams. Clear product labeling helps too; knowing what’s in the bottle gives everyone a fair shot at making safer choices.
Most people won’t think much about the ingredients printed in tiny text on cleaning products. But connecting the dots between a routine chore and a fish kill downriver brings fresh perspective. People with clear information act differently. Regulators need to keep pushing transparency, not just for workers but for all of us pouring these bottles down drains and onto our kitchen counters. Every small choice stacks up — for healthier people and cleaner water.
Step into most cleaning supply aisles, and you’ll notice an arsenal of glass, floor, and surface cleaners promising streak-free shine and scrubbing power. Rarely does anyone squint at the label to spot “propylene glycol butyl ether.” Still, you’ll find it hiding among ingredients because this solvent has become a go-to for cutting through grease without tearing up your nose or destroying surfaces. It’s the type of ingredient that lets you get a window clean in half the time, which matters if you’ve ever needed to tackle spring cleaning or rental turnover in a hurry.
In the industrial world, this chemical quietly pulls its weight. Factories and workshops don’t have time for paint that won’t stick or won’t spread. Propylene glycol butyl ether helps paint and coatings get into all the cracks and corners, letting pigments blend evenly so results look right. Paint that goes on smooth and dries even just makes life easier. My own time spent repainting apartments reminded me what a mess you get from a cheap brush or inferior paint. Quality isn’t always about price; sometimes it comes down to invisible helpers like this solvent.
Printing press operators rely on it too; quick-drying ink means jobs finish up faster. No one wants to wait around as stacks of packaging labels or magazines cure, so a solvent that helps ink set and cling to both paper and plastic makes a day on the line less frustrating.
I’ve worked on both DIY and professional projects—oil stains on patio furniture, sticky adhesives on tools, or restoring an old wooden chair. Heavy-duty cleaning can send fumes through the walls, bringing on headaches or complaints. Propylene glycol butyl ether gives you powerful grease-busting without driving you outside for a breath of air. It doesn’t mean you can shrug off gloves and safety glasses, but the balance it offers between strength and gentleness saves your fingers and your workspace.
No chemical gets a free pass. Research shows that while this ether isn’t as harsh as some heavy-duty cleaners, long exposure could still irritate skin and lungs. I’m no stranger to coughing fits after an afternoon in a locked room with the wrong solution. Knowing ventilation matters hasn’t stopped me from getting lazy on cold winter days, but experience taught the lesson: open a window or prop open a door.
Disposal brings another wrinkle. Dumping leftover solutions down the drain clogs up more than pipes. Water treatment plants struggle enough with household chemicals. Switching to water-based or biodegradable options often helps, but there’s no substitute for reading about safer handling and proper disposal.
Some companies have started tweaking their formulations. It makes sense for health, but also for image. Green certifications now attract more customers. The trick is finding something that works as well as propylene glycol butyl ether without leaving behind a stubborn mess.
Workers, homeowners, and industry all play a role. Smarter labeling, proper education about hazards, and pushing for safer alternatives keep everyone safer. Only by demanding better, both in the products used and the oversight applied, will progress happen—not just for the sake of shiny floors, but for everyone’s health in the long run.
Propylene Glycol Butyl Ether shows up in a lot of products. You’ll find it in cleaning sprays, paints, some inks, and even a few personal care items. It’s there because it helps dissolve grease, break down dirt, and keep things mixed together. For folks with dirty jobs and busy homes, these products offer real convenience.
Every time I read a chemical name longer than two words, I get a little cautious. Who really knows what all those letters and numbers mean? Of course, using chemicals at home or work means trusting that someone out there checked things for safety. Propylene Glycol Butyl Ether doesn’t dodge regulation. The U.S. Environmental Protection Agency and the European Chemicals Agency both keep tabs on it.
Their work points out risks mostly for workers exposed daily, like folks who mix batch after batch of industrial cleaner or use powerful degreasers without much protection. Skin and eye irritation pop up as simple problems, but there’s more. If a person breathes high levels of vapor—especially over time—headaches and drowsiness can show up, and breathing trouble isn’t out of the question.
The average home user rubs way less elbow grease with these products. Typical cleaning routines, done in a well-ventilated kitchen or bathroom, almost never come close to dangerous exposure. The real risk comes from ignoring label instructions, skipping gloves, or spraying willy-nilly in closed-up rooms.
I once watched a friend try to strip old paint using an industrial degreaser in a windowless basement. By the end, his eyes stung and he ended up with a splitting headache. Later we found out he had used a mix that listed Propylene Glycol Butyl Ether near the top of the label. If he had read the directions or cracked a window, most of it could have been avoided. Regular use at home, done right, doesn’t lead to that kind of misery.
History shows that accidents and health scares happen when people skip basic precautions—no gloves, no windows open, tossing around the mop like no tomorrow. My grandparents used similar cleaners for years and never saw big problems, but they always washed their hands after cleaning and didn’t try to turn the house into a chemistry lab.
Clear labeling and better education help everyone. Busy parents, janitors, or anyone scrubbing a stubborn stain can understand gloves and fresh air help. Manufacturers can take things up a notch, offering less harsh alternatives whenever possible or adding stronger warnings. Simple switches—ventilating with a fan, storing chemicals away from kids, not mixing products willy-nilly—make a big difference.
Some workplaces already step up by offering training and better gear for workers handling strong cleaners. Regular home users probably don’t get lessons, but a quick look at the back of the spray bottle goes a long way. People who want a gentle touch can look for green cleaning products that leave out glycol ethers altogether.
Most folks stay safe with Propylene Glycol Butyl Ether just by following the directions and adding common sense to the routine. The real enemy is careless use, not the ingredient itself.
Propylene glycol butyl ether sounds like something out of a chemistry lab, but I keep running into it everywhere. If you’ve ever cleaned your kitchen or painted a room, you’ve probably used products made with this stuff. It's used to help cleaners cut through grease, which makes life a lot easier, especially after cooking a big meal. It shows up in glass cleaners, too, because it tackles fingerprints and dirt without leaving streaks behind. The streak thing always drove me crazy until I learned why some glass sprays work better than others—it's all about this kind of solvent.
I’ve picked up my fair share of paint cans over the years, and the difference between a smooth wall and a patchy one often comes down to what’s in the paint. Propylene glycol butyl ether gets mixed into water-based paints and coatings to help everything spread out just right. As I discovered during a few living room makeovers, paint with better flow means fewer brush marks, and that’s huge when you're not a professional. This chemical stops the paint from drying too fast, letting you fix little mistakes as you go.
Anyone who’s had a child come back from school with art projects knows about messy, bright inks. Printers need solvents to make ink flow without clogging things up or drying at the wrong time. In many workplaces and commercial printing shops, propylene glycol butyl ether plays a quiet part behind the scenes, making sure inkjet printers work efficiently day after day. It makes colors look sharp on paper while helping machines keep up with big stacks of jobs.
Factories use plenty of chemical cleaners to remove oils, grease, and dust from machinery. My friend, who worked in a metal shop, swore by the way these cleaners kept his equipment functioning. Industrial-grade degreasers with propylene glycol butyl ether break up tough grime quickly, keeping downtime low and reducing risk for workers. This solvent does its job without producing harsh fumes that linger, so big workspaces stay safer for people doing shifts inside.
Of course, no chemical comes without some baggage. It’s not a bad idea to crack a window while you clean, because breathing in too much can cause headaches in some people. And just because something isn’t instantly hazardous doesn’t mean it’s harmless at every level. The Environmental Protection Agency keeps an eye on how much of this chemical flows into products. On the consumer side, there’s a growing interest in “greener” cleaners. Some companies have already started swapping out traditional solvents for plant-based ones in household sprays, and shoppers seem eager to try new options.
As more people ask for safer indoor air and fewer surprises in products, it won’t just fall to chemistry experts. Those who make everyday products have a real opportunity to find replacements that work just as well but offer less risk. In my house, I reach for natural cleaners when I can, but I still appreciate strong options for heavy jobs. Switching from chemical-heavy formulas to plant-based choices isn’t always straightforward—sometimes green cleaners just don’t cut it for big messes. But every small choice adds up, and companies that listen to shoppers will probably find plenty of willing customers for safer alternatives.
Propylene Glycol Butyl Ether might show up in the label on cans of paint stripper or heavy-duty cleaners, but it doesn’t carry the everyday familiarity of household bleach. This solvent, clear and slightly sweet-smelling, isn’t the sort of thing you leave sitting on a cluttered shelf in the garage. Anyone who’s worked around industrial chemicals knows carelessness or impatience can turn a simple task into a real headache. Sometimes worse.
This isn’t a chemical to fear, but it does deserve respect. It can irritate skin and eyes, and if you inhale enough vapor, your lungs will let you know right away. Over time, a poorly sealed drum will fill the storage area with fumes, making it unpleasant or even unsafe to stick around.
Those working in factories or maintenance shops often take shortcuts, but stories circulate for a reason. I remember a warehouse manager who shrugged off a small spill in a cramped storeroom. By morning, the sharp odor was all you noticed, and anyone working nearby felt dizzy before noon. The lesson: always seal containers tight and check for cracks or dents before you walk away. Leaks don’t just waste product, they put health at risk.
A sweaty metal can after a summer weekend spells trouble. Sunlight isn’t a friend to propylene glycol butyl ether, and neither are high temperatures. Heat builds pressure inside a sealed drum, stressing gaskets or caps. If those fail, you get unexpected vapor outbreaks. Storing chemicals in a cool, shaded place buys peace of mind.
Ventilation matters more than most people think. Fabricators sometimes park containers in storage closets, thinking it’s safe as long as nobody bumps into them. That stale air traps fumes, turning a mild whiff into a steady, invisible hazard. Even a basic exhaust fan or louvered window will make a difference, pulling stray vapors away before they build up.
I’ve watched new shop workers pile rags, solvents, and paint under a wooden workbench. If one chemical flashes, everything goes up. Propylene glycol butyl ether isn’t as flammable as gasoline, but sparks, open flames, and static charges shouldn’t share a home with any solvent. Store it far from welding gear, water heaters, or piles of oily rags.
Mixing incompatible chemicals can stir up nasty reactions. Oxidizing agents belong on a different shelf. Old habits lead to “all bottles on one rack” organization, but that’s a recipe for a ruined day. Labels wear off, and spills spread quickly. Segregating solvents keeps accidents from escalating in seconds.
Nobody loves doing inventory in the chemicals shed, but lining up containers so that older ones are used first keeps stock fresh and avoids surprises. Routine checks for leaky caps, rust spots, or faded labels save work down the road. Even a minute once a week makes a difference.
Safety gear should hang within arm’s reach: gloves, goggles, maybe a mask if evaporation seems high. Every spill kit needs to be ready to grab, not buried behind empty boxes or old pallets.
Companies with strong safety cultures know that the best accident prevention comes from simple routines and clear communication. Sharing lessons and walking through the process with new team members pays off in the long run. Forget the quick fixes — a few steady habits can spare a business from harm and keep people safe around propylene glycol butyl ether and any similar chemical.
Most folks in cleaning or coatings run into a wide range of chemicals, but Propylene Glycol Butyl Ether (PGBE) shows up more than some might expect. It thins out water-based paints, lifts grease, and has a way of breaking down stains that makes it a regular on the shop shelf. Having spent time in a maintenance shop, I’ve watched enough people treat it like just another colorful bottle. That sort of thinking gets people hurt.
Pop a cap off this stuff without gloves or crack open a jug in a closed room, and before long you can notice the runny nose or the slight headache. Vapor builds up, especially if a room doesn’t have real airflow. In the heat, this even becomes worse. Skin contact leaves irritation—redness or dryness is often shrugged off as ‘just cleaning,’ but over weeks, skin breaks down. Long sleeves and gloves may slow down the work a bit, but they save hands from hours of ache and peeling. Goggles won’t win any contests for style, but nobody plans to flush their eyes at the eyewash station.
Spills happen. I’ve seen coworkers grab coffee rags to mop chemicals off the floor, and by the end of a shift, headaches started lining up. Any splash, no matter how minor, soaks in. The right way to handle spills is to let absorbent pads and gloves do their work, followed up by careful disposal. No one wants trash bins leaking solvent out onto the loading dock.
Storing PGBE means picking shaded, well-ventilated spots—never near food, never near anything with a flame. Labels matter; they keep the next shift from guessing what’s inside an old drum. Even after years around this field, I still double-check anything I’m handling. It's not about forgetting, it’s about preventive habits. The smell alone can’t always tell you what’s mixed in a drum.
Nothing brings a warehouse team closer than a scare with unexpected combustion. PGBE has a flash point, and that warmth in a busy workspace can creep up without warning. Sparks from nearby machinery, faulty outlets, or even stray cigarette butts should keep people cautious. I’ve seen old extension cords create close calls with chemicals like this—avoid them outright.
Mixing chemicals with a ‘what’s the harm?’ attitude only works until it doesn’t. Certain oxidizers or acids make an ordinary job turn threatening. Even mixing with the wrong cleaning agents can churn out more vapor or worse. Check the safety datasheets and read before trying to experiment. The label on a bottle isn’t a suggestion.
Good companies push for regular training for a reason. Even if reading manuals feels dry, acting it out with a team builds habits that help under pressure. Showers and eyewash stations often gather dust—until the one time they’re crucial. Making sure exits stay clear and spills get fixed right away isn’t worrywart behavior. It's community care. Resisting shortcuts is hard, but I’ve seen bandaged hands and red eyes too many times to ignore the cost.
At the end of a long day or night, folks want to step outside and breathe clean air. Nobody wants a sneaky chemical hurting them in years to come. Sticking with all the right precautions, no matter the rush, isn’t just workplace policy—it’s how everyone gets to clock out in good health.
| Names | |
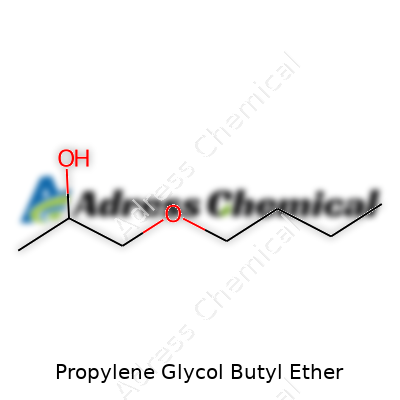
| Preferred IUPAC name | 1-Butoxypropan-2-ol |
| Other names |
Propylene glycol monobutyl ether 1-Butoxy-2-propanol Butoxypropanol PnB Dowanol PnB Propylene glycol n-butyl ether |
| Pronunciation | /ˈproʊpɪˌliːn ˈɡlaɪˌkɒl ˈbjuːtɪl ˈiːθər/ |
| Identifiers | |
| CAS Number | 5131-66-8 |
| 3D model (JSmol) | `C(COC)COCC` |
| Beilstein Reference | 1731589 |
| ChEBI | CHEBI:81313 |
| ChEMBL | CHEMBL1667771 |
| ChemSpider | 11101 |
| DrugBank | DB11123 |
| ECHA InfoCard | 100.011.666 |
| EC Number | 603-052-00-8 |
| Gmelin Reference | 76138 |
| KEGG | C19699 |
| MeSH | D023149 |
| PubChem CID | 7976 |
| RTECS number | UJ0875000 |
| UNII | 947LJ8O5VW |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | 'DTXSID9030677' |
| Properties | |
| Chemical formula | C7H16O2 |
| Molar mass | 118.18 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | mild, ether-like |
| Density | 0.88 g/cm³ |
| Solubility in water | miscible |
| log P | 0.96 |
| Vapor pressure | 0.07 mmHg @ 20°C |
| Acidity (pKa) | 14.8 |
| Basicity (pKb) | 0.60 |
| Magnetic susceptibility (χ) | −8.30×10⁻⁶ |
| Refractive index (nD) | 1.400 |
| Viscosity | 2.5 mPa·s |
| Dipole moment | 2.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.06 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4606.6 kJ/mol |
| Pharmacology | |
| ATC code | D07AX01 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 66 °C |
| Autoignition temperature | 230°C (446°F) |
| Explosive limits | 1.1% - 10.5% |
| Lethal dose or concentration | LD50 (oral, rat): 2,743 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,320 mg/kg (rat, oral) |
| NIOSH | NIOSH: TX 8010000 |
| PEL (Permissible) | PEL: 25 ppm |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 700 ppm |
| Related compounds | |
| Related compounds |
Diethylene glycol butyl ether Ethylene glycol butyl ether Propylene glycol methyl ether Propylene glycol propyl ether |