Ethylene glycol phenyl ether first appeared in chemical catalogues in the early 1900s, but it never made headlines until the middle of the century. As industrial chemistry boomed, chemists started reaching for it when looking for more selective, stable solvents and intermediates. The story of its development mirrors the broader growth of organic synthesis, where every decade brought a new reason to tweak old molecules and find smarter ways to run reactions. Among specialty ethers, manufacturers gave it space for a simple reason: it delivered performance that others couldn’t. The history also shows researchers slowly realizing that its utility extended well beyond solvent applications. Methods of manufacture became cleaner and safer as regulations tightened and as environmental awareness started turning from idea to law.
Most chemical suppliers list ethylene glycol phenyl ether as a colorless to pale-yellow liquid. Its slight, sometimes sweet odor recalls many glycols, but phenyl groups add a twist to volatility and solubility. Chemists often grab it for its rare blend of water-compatibility and ability to dissolve greasy organic compounds. In my experience, its balance of properties can sidestep headaches common with harsher glycol ethers or less selective non-polar solvents. That versatility leaves it open for use as a co-solvent, an intermediate, and a base compound for chemical modifications in the laboratory and in industry.
Among glycol ethers, ethylene glycol phenyl ether stands out for its boiling point around 245°C, which avoids the volatility problems that plague lighter ethers. It flows well even in cold weather, with a melting point below -20°C, and its mild scent never overwhelms a workspace. Water solubility helps when someone needs to mix the hydrophilic with the oily, and it won’t react unexpectedly in most aqueous systems. Density hovers close to 1.1 g/cm³, and flash point usually sits above 110°C, which adds peace of mind in active labs. Chemical compatibility often spares headache, as the molecule resists hydrolysis and most oxidants under standard conditions. Its structure, with an ether and an aromatic ring, makes it stable under heat and slow to degrade in sunlight.
Look at a technical data sheet, and you’ll see most suppliers calling this compound “phenoxyethanol” or “ethylene glycol monophenyl ether,” alongside the more formal IUPAC names. Purity usually clocks in at 99% or above, which makes quality control straightforward for most uses. Acidity stays low, with residual moisture usually listed below 0.1%. Labels must carry proper CAS numbers (122-99-6) and GHS pictograms, highlighting the compound’s mild but real risks. Requirements for safe transport in drums or bulk containers appear in line with glycol ethers of similar profile. Tracking batch numbers and using tamper-evident seals have become standard, as regulatory audits leave little leeway for error, especially for products crossing international borders.
Production often starts with ethylene oxide and phenol, passing them over a basic catalyst like sodium hydroxide. Watching the evolution of these manufacturing methods reminds me that efficiency and safety always compete: older processes generated more waste and required higher temperatures, but modern routes squeeze every barrel for higher yields and leave behind fewer byproducts. Hazardous reagent exposures get limited with improved batch controls and closed-system reactors. Downstream purification, involving vacuum distillation and charcoal filtration, tends to be automated these days. Factory audits make sure the process materials don’t leave behind residues that threaten safety or downstream performance in formulated products.
Ethylene glycol phenyl ether rarely sits idle in synthetic chemistry. Its ether group resists most acids and bases, but strong nucleophiles or oxidants eventually split the molecule. In my work, I’ve seen the molecule go into etherification reactions where the aromatic ring opens up possibilities for further substitutions. Its reactivity inspires formulators to experiment with chain extensions, creating modified ethers for custom surfactants or intermediates. The stability of the phenoxy group reduces side reactions often seen with other glycol ethers. Sulfonation, nitration, and halogenation each bring new performance attributes, opening doors to resins, inks, and specialty polymers. As a mild solvent carrier, the molecule can bring otherwise finicky reactants together, offering homogeneity during the critical stages of synthesis. Tailoring with additional functional groups lets chemists tune solubility and reactivity to suit specific demands.
Walk into a chemical storeroom, and you might spot ethylene glycol phenyl ether under names like “Phenoxyethanol,” “EGPE,” or “2-Phenoxyethanol.” Commercial brands sometimes add their own registered names for signature grades, but the core product remains recognizable through its standard codes. This causes confusion for newcomers, who may not realize that what’s called “phenoxyethanol” in cosmetics is the same chemical as “ethylene glycol monophenyl ether” in paint additives or electronics. The proliferation of synonyms demonstrates how a single chemical can straddle different industries without losing its identity, though it also highlights the ongoing challenge of maintaining accurate inventory and regulatory paperwork.
Handling phenoxyethanol calls for respect but not panic. Spill management often centers on containment and ventilation, as the vapors, though not aggressive, cause irritation with prolonged exposure. The skin-handling guidelines parallel those for most glycol ethers: gloves, goggles, and fume hoods. In larger facilities, air monitoring and regular staff training keep levels in check and reduce incidents. Regulatory agencies, from OSHA to the European Chemicals Agency, keep a close eye on exposure limits in workplaces and finished products, particularly for cosmetics and pharmaceuticals. Waste needs wastewater treatment or incineration with scrubbers to capture aromatic emissions. Emergency response protocols for larger spills stress first aid and decontamination. Safety Data Sheets remain dense, but chemical safety officers often highlight real-world concerns like cumulative irritant effects and long-term environmental fate, underscoring the need for careful management from arrival on site to disposal or recycling.
Few chemicals wear as many hats as phenoxyethanol. In my own lab, it has played everything from solvent to preservative. Paint and ink formulators value its ability to blend water and oil-based components and to boost drying and leveling. Cosmetics and personal care products rely on its bactericidal action, which makes it a go-to preservative. Pharmaceuticals include it in topical solutions and emulsions, counting on its mildness for patient safety. In electronics, it acts as a cleaning agent, disrupting tough residues without corroding sensitive parts. Textile processes rely on it for fiber treatment and dye stabilization, and it appears in specialized lubricants and cleaners. Even as manufacturers spot new ways to put it to work, demand stays steady thanks to its dependable performance across these fields.
Current research leans into green chemistry, searching for methods to produce phenoxyethanol from renewable resources instead of petrochemicals. Teams look at lignin-derived phenols and bio-based ethylene oxide, nudging the industry closer to carbon neutrality. Scientists dissect reaction pathways to squeeze more yield from each bath, using advanced catalysis and reactor designs. In product development, competitors dissect every aspect of purity and impurity profiles to minimize irritation in sensitive users, particularly for baby care and medical products. Researchers explore nano-scale modifications for targeted drug delivery and fine-tune its role as a carrier in specialty polymers. Conferences and journals track these incremental advances, bringing together synthetic organic chemistry and applied sciences for smarter, safer applications.
Most toxicology profiles agree that phenoxyethanol poses low acute oral and dermal toxicity, but repeated or long-term contact brings risks. In my experience, the bigger challenge comes from subtle irritation over weeks rather than dramatic poisonings. Animal studies find effects on the liver and kidney at high doses, spurring industry to put strict limits in personal care applications. Eye irritation remains a consistent finding, so safety glasses still count, even in routine use. Environmental studies show moderate persistence, so wastewater controls play a bigger part than with more biodegradable compounds. Regulatory agencies in the EU and U.S. continually re-evaluate its thresholds as new research on chronic low-level exposure emerges, especially regarding infants and sensitive populations where cumulative risk may matter most.
Looking out ten years, phenoxyethanol will likely become one of the earliest glycol ethers to transition fully into green production. Sustainable synthetic routes from plant-based feedstocks seem within reach, especially as crude oil costs and carbon taxes climb. New regulations will probably split the market between high-purity pharmaceuticals and standard industrial grades, with product innovation riding on advances in purification. Companies rolling out bio-based versions will face tough competition from legacy manufacturers but may win over customers in cosmetics and pharma where traceability matters more. Meanwhile, advances in catalysis, formulation, and waste remediation will keep shrinking the environmental footprint. With R&D on persistent impurities and long-term health tracking, its role in sensitive applications will stay under the microscope, but with careful stewardship, phenoxyethanol stands to remain a staple in labs, factories, and households worldwide.
Ethylene glycol phenyl ether doesn’t sound like something you run across in a grocery store aisle, but folks in manufacturing and chemistry labs see it a lot more often than consumers realize. Its presence pops up behind the scenes, from cleaning products to paints, giving these everyday things a bit of a performance boost. The name might not roll off the tongue, but the stuff inside has been doing some heavy lifting for decades.
The first time I saw the name was scrubbing down beakers after a long day in a university lab. It sits inside many industrial and household cleaners for a reason: it loosens up oily smears and greasy fingerprints where simple soap and water flop. The chemical packs a real punch without tearing up plastic, glass, or painted surfaces. Hospitals, schools, and offices all lean on cleaning supplies that rely on this kind of solvent, trusting it to dissolve grime while leaving floors and benches intact. It means janitors spend less time scrubbing, and facilities bustle on, germ-free and streak-free.
Artists and construction workers owe a lot to solvents; ethylene glycol phenyl ether steps into this world as a dependable co-solvent. Picture a watery paint smearing and never drying right—it’s this ether keeping the pigment mixed, making sure you get even color on every wall or canvas. It slows down drying time, giving amateur DIYers a second chance to fix drips and brush marks. Cans of rust-resistant house paint rely on it for smoothness and spread, and even fancy specialty coatings stay flexible because of its chemical makeup.
There’s real magic in printing presses, and toner wouldn't cling so neatly to slick paper without a helping hand. Printing ink makers mix in ethylene glycol phenyl ether for cleaner lines and faster printing without clogs. Newspapers, labels, cartons, glossy magazines—they gain their sharp colors in part thanks to this solvent. Paper itself sometimes takes a dip in solutions including this ether, making it stronger, brighter, and ready for another round of recycling.
Scan the back label of a hair dye or deodorant spray, and occasionally, you’ll spot this tongue-twister tucked between preservatives and moisturizers. Manufacturers mix it in as a fragrance carrier, making sure floral and musky notes stick around all day. Creams and lotions use it to glide onto skin without leaving a greasy film. This ingredient works in the background, holding ingredients together like an invisible referee, watching over creaminess and shelf life.
Some folks worry about synthetic chemicals in every corner of their home. There’s reason to keep eyes open—government groups like the EPA in the US watch how much of this chemical shows up in air and water. Labs have checked for long-term exposure problems, and most countries set strict limits for workplace air. The big goal: keep workers safe and reduce risks for consumers down the line. Companies explore gentler, plant-based solvents and tighter ventilation, though the speed and cost can trip up even big players.
Chemicals like ethylene glycol phenyl ether highlight the trade-offs in modern production: clean windows, bright magazines, and fresh-smelling lotions, balanced against the push for a safer, greener world. The more everyone asks tough questions about what’s in their stuff, the better the answers will get.
Factory floors, paint shops, and cleaning product plants have seen their share of strange-smelling chemicals, piled up drums, and confusing labels. Ethylene glycol phenyl ether tends to show up anywhere folks need a solvent that can peel away grease or help ink flow smoother. Most likely, the folks working with this stuff rarely call it by name. They just want to get the job done without feeling sick at the end of the shift.
Ethylene glycol phenyl ether doesn’t hide its punch. Eyes and lungs react pretty fast if someone stands too close for too long. People sometimes say their skin starts to sting or itch after splashes. If a worker breathes a lot of the vapors, headaches kick in or they feel dizzy. Longer exposures bring tougher problems. Liver and kidney tests sometimes turn up unusual results after handling this chemical for weeks or months, especially if safety gear gets skipped. Tests on animals have raised concerns about how this solvent affects development and organs over time. Nothing glamorous, just facts from labs that run these chemicals through their paces so workers can get a straight story.
Big regulatory groups don't ignore the health risks. Agencies in Europe slapped on hazard symbols showing serious eye and skin dangers. The U.S. Environmental Protection Agency lists it as a substance with enough risk that companies have to track their handling and make good on worker protections. Plenty of countries demand gloves, masks, and decent ventilation in work areas. Problem is, warnings don’t always carry as much weight when deadlines are tight and someone’s boss isn’t paying attention.
From my time helping clean out printers and paint tanks, fumes drift up fast in a small, closed room. Even a half-hour without fans or a proper mask could mean feeling off for the rest of the day. No one likes donning bulky respirators in the heat, but it beats nosebleeds and months of doctor visits when things get rough. I watched seasoned workers skip gloves once in a while, hoping nothing splashes. Too many landed itchy skin patches that took weeks to calm down. Folks who keep gloves on and find room for airflow usually stick around longer, with fewer sick days.
Ventilation cuts the risk for everyone, not just the person standing right over the bucket. Fans, open doors, and a no-nonsense attitude about keeping lids sealed stop vapors from building up. Choosing the right gloves and eyewear isn’t rocket science; suppliers list what works with each solvent. Proper training goes miles too. Short talks at the start of a shift, quick reminders about safe handling, and stories from coworkers who learned the hard way—these stick better than dry instructions. Substitution matters too. Companies stubborn about hanging onto old solvents might save on upfront costs, but safer alternatives exist. Even though switching formulas sometimes costs more at first, avoiding lawsuits and hospital bills more than pays for itself.
Ethylene glycol phenyl ether asks for attention and respect, not panic. It sits on the shelf like plenty of tools that work hard but bite back when taken lightly. Knowing its risks and staying on top of practical safety habits makes a world of difference for anyone doing an honest day’s work with chemicals.
Step into a lab stocked with solvents and you’re bound to run across chemicals as obscure as Ethylene Glycol Phenyl Ether. Chemists call it 2-Phenoxyethanol, and its formula fits right in with the world of glycols: C8H10O2. Structurally, think of a glycol backbone hooked up to a phenyl ring — easy to draw, a bit harder to explain at a backyard barbecue. This chemical functions in so many ways, from dissolving sticky resins to acting as a gentle preservative.
Pour this liquid out of a brown bottle and what lands in your beaker goes clear. It almost looks like water, though the nose picks up something else: a slight floral odor, almost like rosewater with hints of medicine cabinet underneath. That scent comes from the phenyl group, which also nudges its way into the product label of lotions and soaps. The sensation is unmistakable, even in a room full of other strong chemicals.
Not every solvent makes headlines or sparks panic among parents, but ethylene glycol phenyl ether finds its way into products people trust — vaccines, inks, cosmetics. Safety matters a whole lot here. The compound’s clear appearance does not mean harmless. Touch it long enough every day and skin gets red; spill enough and headaches or worse follow if a person isn't careful. It absorbs through the skin and, over time, could end up in the bloodstream.
As a paint chemist who’s cleaned more than one brush with a splash of this ether, I’ve learned to respect it. Gloves and ventilation are not negotiable. Folks working in printing plants or making lotions rely on this chemical’s ability to mix water and oil, but also need training so no one underestimates its punch. The U.S. Environmental Protection Agency and European safety agencies watch this ingredient closely, especially since old animal studies hinted at toxicity. Limits sit in place because companies have mixed this solvent into baby wipes or vaccine stabilizers for years.
The beauty industry loves 2-phenoxyethanol because it keeps bacteria out of creams without the burn of harsher preservatives. Even so, there’s a sweet spot: enough to stop germs but not enough to leave users with skin rashes. In workrooms, risk grows higher because exposure picks up over the long shift. Here’s where protective culture shines. Good air flow, gloves, clean habits, and real talk about symptoms create safer jobs.
Science keeps looking for alternatives, though chemistry is a stubborn field. Swapping out a solvent for a safer option doesn’t happen overnight, because the properties that make ethylene glycol phenyl ether useful — blending power, stability, gentle scent — aren’t easy to duplicate. Some labs lean on newer preservatives or plant-based solvents, but replacing this one on an industrial scale runs into cost and performance barriers. That said, regulation matters most when the chemical could end up on a newborn’s skin or inside a vaccine vial.
People thrive in a world where chemicals work for us, not against us. Ethylene glycol phenyl ether, with its innocent look and complex reality, makes its mark on formulas and lives. Workers and consumers both deserve clear rules, solid training, and a fair shot at safety.
Anyone who’s ever worked in a laboratory, a warehouse, or a manufacturing plant knows that chemical safety isn’t just about rules on a sheet or labels stacked on bottles. It’s about daily habits, real risks, and the reality that mistakes affect lives and livelihoods. Ethylene glycol phenyl ether, with its mild odor and transparent look, can give off a misleading sense of simplicity. There’s a catch: This compound calls for a real sense of respect and a no-nonsense approach to how it’s stored and handled.
Keeping containers sitting open or stashing them near heat sources doesn’t just risk product spoilage—it sets up the scene for health hazards. Even a brief skin contact with ethylene glycol phenyl ether may trigger redness or dryness. A careless spill, left unchecked, exposes others down the line. With inhalation or long-term exposure, problems only multiply. It’s easy to think “nothing has happened to me yet,” but slow risks add up over time. Routines that shy away from shortcuts will always win out.
Solid storage starts with location. Choose a spot away from sunlight and direct heat. Metal shelves or non-reactive cabinets work best. A dry spot, well-ventilated, blocks vapor buildup and lowers the risk of container corrosion or unexpected leaks. Keep the container firmly closed, using correct seals or screw caps. Label everything—legibly, without shortcuts or fading ink. In my early years working in a storeroom, I watched someone grab what looked like a water bottle from the wrong shelf. The label had worn off, and the mix-up could’ve led to a disaster.
Opening up a bottle isn’t just a hand-and-eye job. Gloves make a world of difference. Choose nitrile or neoprene, because latex can let certain chemicals through. Splash goggles and long sleeves should become as routine as grabbing your wallet before heading out the door. Nobody wants to learn what a chemical burn feels like. Respirators matter, especially in spots with poor airflow—simple cotton masks won’t cut it. Ventilation, real and mechanical, stands between you and accidental inhalation.
Getting rid of waste isn’t something to handle on autopilot. Pouring leftover material into the sink or trash creates a headache for plumbing, the environment, and anyone else handling waste streams. Instead, line up dedicated waste containers, marked with clear labels, away from food or personal items. Most local regulations dictate where and how to dispose of chemical waste, so cutting corners here invites more problems than it’s worth.
Accidents can catch anyone off guard. That’s why emergency wash stations—functional and clearly marked—make a difference when seconds matter. I remember a colleague stumbling after a splash while handling a transfer. Access to an eyewash station right there spared him permanent harm. Emergency protocols might sound repetitive, but the real thing happens fast. Training counts. Dry runs with staff turn mechanical responses into second nature. Every small act—keeping an aisle clear, doing quick checks, logging details—keeps everyone a bit safer each day.
Handling and storing ethylene glycol phenyl ether isn’t complicated science, but it calls for consistency and respect. The routines we set today make tomorrow safer for every single person who walks into the storage area or production floor.
Ethylene glycol phenyl ether may not ring a bell for most people. It doesn't replace the awe of new tech gadgets or grab attention like electric vehicles do, but industries wouldn’t function smoothly without compounds like this. I remember walking through the maintenance area at a printing plant, catching that familiar chemical note in the air, and realizing just how much this one ingredient runs behind the scenes.
Printing companies lean heavily on ethylene glycol phenyl ether, usually calling it by one of its trade names. It helps inks spread the right way on paper or plastic films and prevents them from drying out on press machinery. Years ago, working as a technical consultant, I saw repeated headaches when low-quality substitutes gummed up printheads, ruined whole press runs, and cost thousands. This chemical keeps things running and ensures colors don’t dry mid-run, saving both money and hassle.
Factories and workshops keep ethylene glycol phenyl ether handy for cleaning and degreasing. It breaks down oily films and stubborn residues without corroding most surfaces, making it a staple for engineers who maintain heavy equipment. Car repair shops, electronics assembly houses, and even hospitals trust it to wipe away contaminants. The demand for high standards in electronics makes strong solvents like this one very important—soldering residue doesn’t stand a chance—and that reduces system failures later on.
This might come as a surprise, but it's also in personal care. Big cosmetic brands often use it in formulations for creams, lotions, and cleansers. The reason is simple: it dissolves perfumes and oily ingredients better than plain water or alcohol-based systems. Visit any supermarket and pick a bottle of hand lotion from the shelf—chances are it contains a clever blend of solvents, including this one, to keep it smooth and stable. Concerns over skin sensitivity pop up, but studies show it’s less harsh than stronger, more aggressive solvents.
Farms, orchards, and golf courses spray hundreds of different treatments each year, and ethylene glycol phenyl ether appears on many of those ingredient lists. It helps dissolve active ingredients so they move smoothly through spraying equipment and spread well over leaf or soil surfaces. This makes every drop count. During a stint helping an agricultural research team, I noticed that using better solvents meant crops absorbed more of the treatment and fields required fewer repeat applications, cutting costs and lowering runoff into waterways.
Textile manufacturers use this chemical to help dye penetrate fibers and finish fabrics. Without sturdy solvents, dyes streak or fade, and customers complain. My family worked in upholstery for years, and they’d often grumble about fabric runs where the colors just didn’t hold up. Understanding what goes into the chemistry behind the scenes gives you new respect for why your couch stays bright year after year.
Chemical safety draws more attention now than twenty years ago. Ethylene glycol phenyl ether doesn’t carry the severe risks of some solvents, but proper handling and storage matter. Green chemistry’s rise means more companies look for plant-based or less-toxic alternatives, but practicality and price still give this compound a foothold. I’ve seen factories test supposed replacements, only to switch back after production hiccups or customer complaints. It’s a reminder: sometimes, humble workhorse chemicals keep industry ticking in ways most people never see.
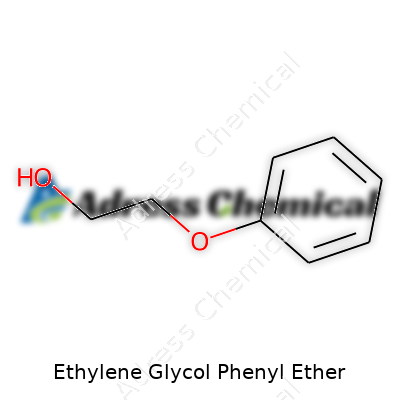
| Names | |
| Preferred IUPAC name | 2-Phenoxyethan-1-ol |
| Other names |
2-Phenoxyethanol Ethylene glycol monophenyl ether Phenoxyethanol Phenyl cellosolve Glycol monophenyl ether |
| Pronunciation | /ˈɛθ.ɪ.liːn ˈɡlaɪ.kɒl ˈfiː.nɪl ˈiː.θər/ |
| Identifiers | |
| CAS Number | 122-99-6 |
| 3D model (JSmol) | `/X2hIOQcGkwYy6Krw1FAFh_model.jmol` |
| Beilstein Reference | 604984 |
| ChEBI | CHEBI:82166 |
| ChEMBL | CHEMBL32084 |
| ChemSpider | 11816 |
| DrugBank | DB14184 |
| ECHA InfoCard | 100.041.257 |
| EC Number | 204-993-3 |
| Gmelin Reference | 6727 |
| KEGG | C06443 |
| MeSH | D004982 |
| PubChem CID | 7518 |
| RTECS number | KN0175000 |
| UNII | 8I3SC2EE85 |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C8H10O2 |
| Molar mass | 138.17 g/mol |
| Appearance | Colorless liquid |
| Odor | Faint aromatic odor |
| Density | 1.102 g/cm³ |
| Solubility in water | Partially soluble |
| log P | 1.18 |
| Vapor pressure | 0.02 mmHg (20°C) |
| Acidity (pKa) | 15.2 |
| Basicity (pKb) | 15.09 |
| Magnetic susceptibility (χ) | -63.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.530 |
| Viscosity | 11.5 cP (25 °C) |
| Dipole moment | 2.34 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 336.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -294.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3844 kJ/mol |
| Pharmacology | |
| ATC code | D08AX04 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | **GHS02, GHS07** |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P321, P363, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 Health:1 Flammability:2 Instability:0 |
| Flash point | 204°C (399°F) |
| Autoignition temperature | 233 °C (451 °F; 506 K) |
| Explosive limits | Explosive limits: 1.1–9.6% |
| Lethal dose or concentration | LD50 oral rat 1610 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (oral, rat) |
| NIOSH | MN9275000 |
| REL (Recommended) | 2.5 mg/m3 |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol Phenyl ether 2-Ethoxyethanol Propylene glycol phenyl ether |