Long before the era of smartphones and constant chemical innovation, chemists hunted for solvents that could dissolve tough coatings without corroding metals or creating fire risks. Ethylene Glycol Monobutyl Ether Acetate (often listed as EGBA or Butyl Cellosolve Acetate) first crossed from research papers into factories in the mid-20th century. People in the paint and coatings business wanted something that could thin tough enamels without leaving strong odors or sticky residues. Companies, particularly in the US and Europe, rolled out this compound to tap into post-war construction and auto booms. Over time, as regulations on volatile and flammable solvents tightened, EGBA picked up more market share due to its balance of solvency and manageable toxicity.
Most folks see this chemical as a colorless, almost odorless liquid. It doesn’t turn heads in the lab, but industry teams care less about looks and more about performance. Bottles and drums labeled with its IUPAC name or aliases, like 2-butoxyethyl acetate, find their way into everything from automotive paint lines to electronics cleaning. Easy to pump and measure, it fits straight into automated mixing systems. One aspect that helps is its moderate evaporation rate—fast enough to avoid sticky surfaces, slow enough to ensure even application. Out on the shop floor, that means fewer mistakes and less waste.
This liquid carries a molecular weight of 176.2 g/mol, slips into a boiling range between 192°C and 196°C, and flashes at around 69°C. It mixes well with most organic solvents, but water beads on its surface due to its limited miscibility. The vapor is heavier than air, which matters in workplace ventilation planning. Despite the faint fruity odor, it’s not aggressive enough to overpower a lab or factory space. Density hits about 0.94 g/cm³, offering easy calculation for bulk users, and viscosity sits at 2.8 mPa·s at 25°C, which proves handy for those shaping the flow rates in spray processes.
Labels sport more than just its chemical name; you’ll also spot hazard pictograms warning about irritation to eyes and skin and flammability under odd conditions. SDS sheets lay out the nuts and bolts: purity, water content limits (often below 0.1%), and distillation range. Purity usually runs above 99%. For shipment, the dye and coatings sector mostly sticks to drums and IBCs (intermediate bulk containers), each stamped with UN numbers and hazard classes per international regulations. Everyone from warehouse staff to high school chemistry students can tell at a glance this isn’t a substance you leave open on the bench.
Chemical plants typically synthesize EGBA by esterification of ethylene glycol monobutyl ether with acetic acid or acetic anhydride, using an acid catalyst. This process churns out the acetate ester along with water. To separate the product, engineers rely on vacuum distillation, so minor byproducts like higher homologs or unreacted glycol ether get filtered out. Factories stick close to closed-loop systems to wring out as much usable product as possible, both for cost control and environmental compliance.
EGBA is not just a solvent; it also reacts with strong acids and bases. The acetate group can hydrolyze in alkaline conditions, reverting to ethylene glycol monobutyl ether and acetic acid. Over time, especially if stored poorly, the product can absorb moisture and slowly degrade—something I’ve seen firsthand in old warehouse stocks, where improper seals led to off-spec material. As an acetate, this ether combines with other functional groups in multi-step syntheses, giving it a place in the chemical toolbox beyond paints and coatings.
Open a catalogue and the synonyms roll out: 2-butoxyethyl acetate, butyl cellosolve acetate, EGBEA, and CAS number 112-07-2. European formulations might use EINECS 203-933-3, while suppliers in Asia go for names like BGA or butyl glicol acetate. This diversity in naming has tripped up more than one procurement officer or lab technician. Even within a single company, warehouse logs might list three or four product codes, proving the need for solid cross-references.
Staying safe means more than gloves and goggles. Short-term exposure may irritate skin or eyes, while inhaling vapor can spark headaches or dizziness. OSHA limits workplace air concentrations, and companies install direct venting and spill traps to avoid chronic exposure. Spill kits on the production floor carry absorbent pads and neutralizing solutions. If you ever try to ship or truck this chemical, international rules require clear labeling, and drivers need safety training. Long stories from plant managers remind us that lapses—such as a leaking joint or skipped air monitor check—lead to expensive cleanup and lost production time.
EGBA shows up where strong solvency meets gentle handling. Factories use it in automotive and appliance enamels, inks, and even printing presses to keep colors smooth. The electronics industry relies on it as a cleaning fluid for delicate circuit boards because it removes flux and residues without damaging sensitive components. More than once, I’ve watched operators choose EGBA over faster-evaporating solvents for touch-up work that demands a perfect finish. A handful of art-supply producers add it to specialty varnishes for murals and sculptures, enjoying its slow drying time for large-scale pieces.
Scientists today remain focused on cheaper, safer production along with biodegradable alternatives. Synthetic chemists keep working on greener catalytic systems that use less energy and cut byproducts. In the coatings sector, material scientists look for new blends that pack high performance but limit inhalation risks. Bio-based routes using renewable feedstocks are picking up steam, not just as marketing fluff, but as a hedge against supply shocks or new regulatory hurdles. Collaborative projects, linking academic research with private industry, have sparked some neat pilot plants and patent filings.
Decades of studies have shown that EGBA poses moderate health hazards at high exposure. Animal tests and occupational exposure reports build a complicated picture—short bursts can irritate, long-term contact raises concerns about blood disorders or kidney effects. Labs have run repeated-dose studies to map out thresholds and chronic symptoms. A string of controversial findings from the ‘80s led workplaces to tighten controls and install vapor monitoring. My own conversations with plant medics confirm that real-world incidents often stem from a lack of training or rushed clean-ups rather than pure chemical risk. Ongoing work aims to clarify the effects of trace exposures, especially where workers might face mixtures of solvents.
The chemical doesn’t look set to disappear, though competition from bio-based solvents looms. With tightening regulations on volatile organics and stronger demand for safer consumer goods, manufacturers will likely trim usage or develop modified forms with lower volatility. Start-ups already talk about closed-loop recovery and recycling services for large solvents users, taking cues from battery recyclers or specialty waste handlers. As digital sensors spread across plants, real-time tracking of vapor and leak rates means fewer accidents, less guesswork, and more data to share with regulators. If research into green chemistry pays off, next-generation materials might blend the strengths of EGBA with improved pollution control, pushing the solvent world toward safer working environments without compromising product performance.
Factories and workshop floors across the world rely on a long list of chemicals most of us never notice. Ethylene Glycol Monobutyl Ether Acetate, often known as Butyl Cellosolve Acetate, turns up in places a lot of folks wouldn’t expect. If you’ve ever painted a wall or bought a product with a glossy coating, there’s a fair chance this ingredient played a part in it. Big paint companies count on this stuff because it helps their paints spread easily and dry evenly, avoiding clumps or streaks. Furniture makers, car plants, and even companies making electronics reach for this chemical to get smooth, professional surfaces on what they build.
Let’s be honest, nobody thrills at paint fumes. But the right ingredients can make a painter’s job quicker and cut down on harsh smells. Butyl Cellosolve Acetate keeps the paint flowing at just the right speed. Instead of paint drying too fast, sticking awkwardly, or leaving rough patches, workers get more time to make everything look just right. Surfaces like car bodies or kitchen cabinets need a smooth, gleaming finish, not a mess of brush marks. This solvent gives industries steady results that last.
If you work with adhesives or printing inks, you’ll run into this chemical too. It’s great for dissolving other tough ingredients, blending them into a mixture that holds well and sits smooth on paper, fabric, or plastics. On big industrial presses, operations need a chemical that keeps inks wet just long enough for a crisp transfer before drying at just the right speed. Most office workers never think about what makes printed brochures or labels look sharp, but processes like this depend on reliable compounds that don’t cause costly hang-ups.
Here’s a tricky bit—while this chemical offers real-world solutions, it does mean exposure risks for the folks who work closest to it. Breathing in the fumes or letting it touch skin over and over can be a problem. Reports out there link repeated contact to headaches, dizziness, and in some cases, trouble for the liver or kidneys. In tight, unventilated spaces, workers start to feel the impact sooner. That means safety gear and keeping doors or windows open turns into a must, not just a “nice to have.” For me, learning about all these behind-the-scenes substances scratched an itch to actually look deeper at what ends up in everyday products. Regulations help shape how safely these chemicals get used, but gaps show up—especially in shops that cut corners or skip safety basics.
Putting safety up front often feels like an extra step. Still, looking at how some places treat their crews, making room for better training and equipment makes a world of difference. Chemical-resistant gloves, face masks, even basic ventilation fans—these don’t cost much compared to a workplace accident or chronic illness. Long ago, I saw a crew painting railings in a building with hardly a cracked window. After one worker keeled over from the fumes, they changed their habits fast. It’s not only about big factories—small shops and home renovators need to hear the message, too.
Switching to lower-toxicity alternatives counts where possible, but industries move slow. Honest labeling and guidance mean regular people get the best shot at protection when handling these liquids at home or on a job site. The more conversations we have about what’s really going into paints, inks, and finishes, the safer the workspace can become for everyone involved.
Working with chemicals in any setting brings a certain risk, but ethylene glycol monobutyl ether acetate stands out due to its wide use in paints, coatings, inks, and cleaning products. I’ve worked in a paint shop and learned quickly that this solvent can seem harmless—clear liquid, mild smell—but exposure takes a toll if you’re not prepared. Eyes, lungs, skin, and even the liver can suffer from careless handling.
This solvent enters the body through skin absorption and breathing the vapors. Unlike a splash of water, even brief skin contact may strip oils, causing dryness and irritation. Prolonged exposure gets worse—it has caused headaches, dizziness, and, in rare cases, a hospital visit from a co-worker who ignored persistent nausea. Breathing in heavy concentrations in poorly ventilated areas can leave folks foggy-headed, tired, and worse. The chemical has also been linked in studies to red blood cell effects if used without safeguards over the long term.
My own mistakes taught me that a T-shirt and jeans aren’t going to cut it in a shop full of solvents. For this one, gloves rated for chemical resistance (nitrile, neoprene), long sleeves, and goggles provide real coverage. Standard safety glasses leave gaps—fully sealed goggles keep splashes out. I keep a good set of gloves nearby, tossing them when any cracks or discoloration appear. Heavy-duty aprons give peace of mind when splash risk goes up. Respiratory protection becomes important if ventilation lags behind. Even a half-mask respirator with organic vapor cartridges keeps headaches at bay during busy shifts.
Cramming into a closed room with paint thinners running is a recipe for trouble. Mechanical ventilation—exhaust fans and open windows—keep air moving and contamination low. I’ve seen makeshift setups where a single fan made all the difference. No one wants to read a safety data sheet every day, but understanding vapor limits and keeping exposure well below them is basic shop smarts.
It’s easy to overlook that a little spill can fill a small room with fumes in minutes. Straightforward habits work best. Clean spills right away with absorbent pads, and hold that rag waste in dedicated barrels. Containers deserve careful labeling—never trust a mystery jug, even for a day. I once saw a water bottle reused for leftover solvent, which nearly led to someone drinking the wrong liquid by mistake. Locked, cool, and ventilated storage protects people and avoids accidental ignition around sparks or heat.
Shortcuts don’t pay when solvents cause health problems you can’t see coming. The right protective equipment, honest attention to airflow, fast cleanup, and “no exceptions” labeling make the difference. We owe it to ourselves—and our co-workers—to change gloves at the first tear, double-check a face mask, and keep work spaces sharp. My experience proves that these habits directly lower risk, day in and day out.
Ethylene glycol monobutyl ether acetate sounds like something only a chemist would worry about, but the truth is, it's everywhere. This isn't just a lab chemical. Painters use it, manufacturers use it, even people refinishing furniture at home get exposed to it. It shows up in certain paints, coatings, and cleaners, quietly doing its job to dissolve or thin stubborn materials.
Ask anyone who’s painted rooms without cracking a window—bad air gets to you fast. Ethylene glycol monobutyl ether acetate really can irritate your nose and throat. It’s not a mild cough, either; it can sting your eyes, dry out your skin, make your chest feel strange. I once helped a friend strip old varnish off wood doors, and despite wearing gloves, my hands felt raw for days. OSHA and the EPA both list this chemical as potentially hazardous not because they want to scare people, but because they’ve seen how it affects workers over time.
Longer exposure takes it up a notch. Factory employees or house painters using products with ethylene glycol monobutyl ether acetate day in and out face real hazards. Researchers have linked repeated inhalation or long-term skin contact to liver and kidney problems. There’s a particular concern for reproductive health—several studies in animals show damage when they’re exposed to high amounts. Humans aren’t mice, but the red flag is up. Even low levels can trigger headaches or dizziness. Once, during a weeks-long renovation, a neighbor complained about nausea and a metallic taste in her mouth. Later, she learned her “eco-friendly” stripping gel still had this ether in it.
This is no reason to panic and avoid every can labeled with complicated names. But a wise approach matters. Relying on strong ventilation isn’t just good advice, it’s a lesson learned the hard way for many people. I now keep windows wide open, run fans, and take breaks outside whenever I’m cleaning or painting. Gloves and goggles these days aren’t overkill. I’d always rather answer, “Why are you all geared up?” than deal with burned eyes or cracked skin for weeks.
Regulators set limits for chemicals like ethylene glycol monobutyl ether acetate. The problem? Rules can’t catch everything, and not every workplace or DIYer follows all the recommendations. Respirators and fume hoods in factories do protect workers, but the patchwork approach to home safety stays risky. Labels could help more—the fine print on most products just doesn’t jump out at people who aren’t chemical engineers.
It starts with ditching assumptions. Just because a product is sitting on a store shelf doesn’t mean it’s safe for unlimited use. It also helps to stop thinking of gloves, goggles, and open windows as fuss. These are basics, not optional extras. If schools spent more time teaching real-world chemical safety, maybe fewer people would experience that unforgettable stinging or headaches from an afternoon’s project.
Looking ahead, it makes sense to keep pushing paint and cleaning companies to use alternatives. Some already cut out risky solvents for water-based ones, and consumer pressure moves this along faster than endless studies. The goal shouldn’t just be about lowering risks for those spending all day with solvents—it should be about making sure anyone tackling a paint job at home can breathe easy, literally and figuratively.
Ethylene Glycol Monobutyl Ether Acetate isn’t a chemical folks talk about at the dinner table. In workspaces though—especially where paints and coatings roll out by the drum—knowing its boiling and flash point matters. The boiling point comes in at about 192°C (378°F), and the flash point, where its vapors can catch fire, hits around 72°C (162°F) by closed cup measurement. To put it all on the table, these numbers don’t just fill labels; they shape the entire approach to handling, storing, and using the solvent.
The boiling point tells you just how much heat the stuff can take before it starts turning into vapor. Once that happens, the game changes. Rooms fill with fumes faster, ventilation becomes more critical, and long shifts near the tanks start to feel different. Walking by an open drum on a hot day, it takes only a gentle breeze to get the smell. For people using this in an auto body shop or a factory, the boiling point isn’t trivia—it’s a guardrail.
That flash point is a warning taped right onto the can. At 72°C, you don’t need a roaring fire to start trouble. In a summer warehouse, sunlight alone could push surfaces dangerously close. I’ve met folks who never saw themselves as risk-takers until they left a container near a heat vent. A fine vapor cloud doesn’t give you any notice; a spark from static, a motor, or old wiring does the talking. The stories behind fire codes often spring from afternoons working with solvents just like this one.
High boiling means spills soak into surfaces and linger, giving off vapors longer. Fragrance lingers too, but this isn’t perfume—these vapors add to indoor air load. Over time, headaches and sore throats can become routine unless fans, hoods, and regular breaks step in. Flash point puts pressure on both storage and disposal. Flammable liquids live under strict rules, and the label on this drum falls in the caution zone. Even a little leftover in a rag makes a difference. Safety cabinets turn from a legal box to check into a real shield.
Nobody wins by cutting corners on chemical storage. I’ve seen shops where fork trucks and open cans mixed with summer heat, and it only takes one bad combo. Get the ventilation going—it’s not a luxury. Store containers away from sunlight and machinery. Train everyone on getting lids back on right away, even during lunch breaks. Ground the containers when pouring, so static won’t start a fire. Use explosion-proof switches where drums stay. For spills, stick to absorbent pads meant for solvents and get them into closed waste cans fast.
Good safety plans always come down to routine habits, not big announcements. Everything from properly labeling the drums, maintaining spill kits, to planning escape routes if things go sideways—these steps pay off. The boiling and flash points might look like a couple of numbers until you’re sweating through a shift, surrounded by cans and the hum of machinery. Then, those numbers become the difference between just another workday and a story you’ll never forget.
Ethylene Glycol Monobutyl Ether Acetate isn’t a name folks hear often outside paint shops or chemical plants. It’s a key part of many cleaning agents and coatings. I’ve spent enough years working with similar solvents to know that the way we store and move these liquids can decide if a business runs smoothly or faces major headaches. Mishandling this material doesn’t just mean a wasted product—it brings real health and safety risks to the people doing the job every day.
Any worker who’s opened a drum of this acetate knows the smell, and many remember warnings about what happens if vapors linger. I’ve seen people toss containers into a corner of a stuffy warehouse “just for now,” only to regret it after noticing warped plastic, leaking drums, or headaches from fumes. Storing this material out of direct sunlight keeps heat from building up and raising vapor pressure. Unless you want to chase leaks, use steel drums or tightly sealed metal containers—not old plastic buckets. Floor spills turn slippery fast, so dedicated racks or storage bays built to catch drips go a long way in keeping things safe and clean.
Humidity sneaks up in many places I’ve worked, so keeping the area dry and well ventilated helps avoid rust and mold, both of which can damage labels and seals. I remember fighting rusted storage racks more than once in an old shop on the Gulf Coast. Moisture shortens the shelf life of this chemical and turns a simple storage room into a costly cleanup site. Simple fans and dehumidifiers aren’t fancy, but they work.
Plenty of accidents start with the phrase, “I thought this was something else.” Make labels big and bold—no one wants to squint and guess. It’s not about rules for the sake of rules—labels save a ton of confusion, especially in busy warehouses. In my early days, faded marker on a drum meant we once mistook a solvent for something harmless. Now, using waterproof labels, color coding, and bold warnings is a lesson learned the hard way.
Loading these chemicals for delivery seems straightforward. Having sat through safety briefings and seen more than a few spills, I can say that most trouble comes from rushing. Trucks and forklifts deserve chemical-proof liners. Drivers need clear routes and should avoid potholed roads that can rattle containers loose—during one delivery job, I watched a poorly strapped drum leak onto other cargo just miles from its destination. The cost wasn’t just lost product; a crew spent hours scrubbing and ventilating the truck. Using air-tight caps and double checks for every load pays off over time.
Some think transport insurance and paperwork matter only to the office folks, not the drivers or loaders. That attitude flips quick when the wrong paperwork holds up shipments or doesn’t have emergency contacts during a spill. Regular drills and access to spill kits mean workers act fast, not frozen, when trouble starts.
After years in warehouses, I’ve seen policies pinned to corkboards, usually ignored. The difference comes down to training you can use, not just memorize. Give workers a chance to use spill kits, practice using respirators, and handle drums with the same tools they’ll have on the job. Accountability looks like checklists and supervisors who actually walk around and talk to the team—not just fill out forms.
The way we store and move Ethylene Glycol Monobutyl Ether Acetate shapes the whole operation. Spills ruin more than product—they risk lives and sour relationships with neighbors, regulators, and customers. Practical, daily habits keep everyone safer and business out of the news for the wrong reasons.
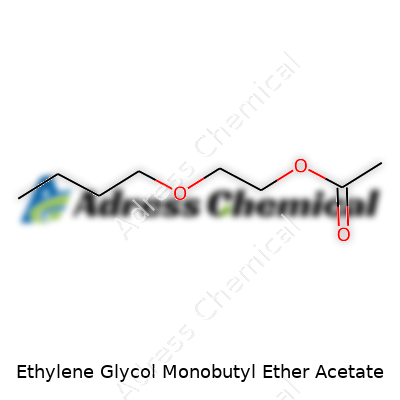
| Names | |
| Preferred IUPAC name | 2-butoxyethyl acetate |
| Other names |
2-Butoxyethyl acetate Ethylene glycol butyl ether acetate Butyl cellosolve acetate EGBEA Glycol ether EB acetate |
| Pronunciation | /ˌɛθ.ɪˌliːn ɡlaɪˌkɒl ˌmɒn.oʊˈbjuː.tɪl ˈiː.θər əˈsɛɪ.teɪt/ |
| Identifiers | |
| CAS Number | 112-07-2 |
| Beilstein Reference | Beilstein 1751478 |
| ChEBI | CHEBI:81337 |
| ChEMBL | CHEMBL135598 |
| ChemSpider | 77321 |
| DrugBank | DB14182 |
| ECHA InfoCard | 03b79b65-e6e2-43e5-9344-3df7206f2b62 |
| EC Number | 203-933-3 |
| Gmelin Reference | 82194 |
| KEGG | C19504 |
| MeSH | D017225 |
| PubChem CID | 12236 |
| RTECS number | KJ9100000 |
| UNII | E1VKO2F56Y |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C8H16O3 |
| Molar mass | 190.24 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild ester-like |
| Density | 0.942 g/cm³ |
| Solubility in water | Soluble |
| log P | 1.61 |
| Vapor pressure | 0.3 mmHg @ 20°C |
| Acidity (pKa) | pKa ≈ 15.5 |
| Basicity (pKb) | pKb: 4.6 |
| Magnetic susceptibility (χ) | -48.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.419 |
| Viscosity | 1.0 mPa·s (at 20°C) |
| Dipole moment | 3.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 410.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –589.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4207 kJ/mol |
| Pharmacology | |
| ATC code | D07AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H312, H332 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 68°C (154°F) (closed cup) |
| Autoignition temperature | 230°C |
| Explosive limits | 1.1–10.6% |
| Lethal dose or concentration | LD50 (oral, rat): 2400 mg/kg |
| LD50 (median dose) | 2,400 mg/kg (rat, oral) |
| NIOSH | KCE |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | IDLH: 700 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol Ethylene glycol monobutyl ether (2-Butoxyethanol) Ethylene glycol monoethyl ether acetate Ethylene glycol monomethyl ether acetate Diethylene glycol monobutyl ether Propylene glycol monobutyl ether acetate Butyl acetate |