Society’s hunger for smarter materials hit a tipping point all through the 20th century, especially after world wars amped up the chemical industry’s ambitions. Ethylene Glycol Methyl Ether Methacrylate popped up in the late half of the last century, growing out of demand for resins and polymers with greater flexibility. Laboratories needed something more tuned than standard methyl methacrylate, and chemical companies responded by expanding their toolkits. What started as a lab curiosity spread, shaped not only by academic papers but by the pull from electronics and coatings manufacturers who always want lighter, tougher, more precise components. In my view, these stories don’t get enough attention—chemists in small, fluorescent-lit rooms have provided plenty of the nuts and bolts that modern life quietly relies on.
This monomer serves as a foundation for advanced copolymers, frequently used where both flexibility and chemical resistance matter. Not everything gets built up from scratch every day—and this is a fine example. Instead of inventing a wheel, companies often tweak the methyl group, stir in an ether, and find that old methyl methacrylate becomes a whole new building block. I’ve seen manufacturers turn to this molecule for specialized adhesives, impact-resistant coatings, and sophisticated acrylate blends. Its history lines up with the bigger story in chemistry: push the envelope, keep tweaking, find better outcomes for both everyday and cutting-edge jobs.
You open a bottle of Ethylene Glycol Methyl Ether Methacrylate and notice a clear, slightly viscous liquid. Its faint, sweet odor can tip off anyone familiar with ethers. This monomer brings a molecular weight just north of 188 g/mol, a boiling point that clears 180°C, and enough volatility to demand good ventilation. Solubility in water stands out—unlike older methacrylate esters, this one dissolves well, which gives it an edge for applications leaning on water-borne systems. Free-radical polymerization feels easy here: a pinch of suitable initiator will start curing, even at mild temperatures. If you work in a lab or plant, these details matter—they steer everything from storage routines to safety checklists, and influence the kind of creative chemistry you can try.
Reading a product label gives you a snapshot of technical culture as much as a chemical’s pedigree. For Ethylene Glycol Methyl Ether Methacrylate, the typical label shows purity at 97% or higher, often lists inhibitors like MEHQ to fight premature polymerization, and assumes a CAS number—2370-63-0. Specific gravity tends to run around 1.08, which means a jug feels about like a similar-sized container of syrup. Viscosity falls in line for its class: thin enough to pump, not so free-flowing you spill it with every move. Labels have to spell out hazard class (flammable liquid, skin and eye irritant) and, more usefully, clear handling guidance. Nobody wins if a technician misses an inhibitor warning or skips a splash guard when decanting for a pilot batch.
Manufacturers churn out this monomer through an esterification route, bringing together methacrylic acid with ethylene glycol methyl ether under acid catalysis. A dehydration step draws off water, and distillation follows to purify the product. I’ve watched familiar reaction vessels bubble away for hours in pilot plants—control is key since runaway reactions or contamination can lead to off-spec batches or worse. Engineers debate columns and resin beds, and there’s always some tension coiling around yield versus purity, day after day. Each time a new licensor comes along, the process tweaks: swap an acid catalyst for a solid resin, or dial in the ether feed. Every step upstream shapes the downstream performance, whether you’re making adhesives or pushing out samples for a university collaborator.
This monomer plays well in radical polymerization, serving as a backbone or branch in acrylic-based materials. Copolymerization teams it up with acrylates, vinyl esters, or styrenics, tuning flexibility or adhesion to fit the user’s need. I’ve seen modifiers introduced through simple co-reactions; epoxy groups or hydrophilic segments can get stitched in, unlocking potential for medical hydrogels or advanced coatings. The ether group opens doors for more nuanced chemistry, letting you chase amphiphilic or UV-reactive segments for things like photoresists or antistatic films. Every advance in formulation highlights the molecule’s role as both a workhorse and a creative tool, supporting tomorrow’s ideas.
Paperwork trails and procurement checks often mask this compound behind alternate names: 2-Methoxyethyl methacrylate shows up often, along with EGME methacrylate. Major suppliers print their proprietary trademarks, but the key numbers—CAS and EC—make sure you’re buying what you want, not a confusingly similar sibling. I’ve seen engineers scan MSDS sheets for errors, only to find regional synonyms or branding differences. So double-check the label before a new batch enters the storeroom, because swapping in the wrong ester means headaches at best and process aborts at worst.
Open this monomer in a warm shop and the vapor finds its way to your nose quick. Gloves—standard nitrile—are a must, and splash goggles never feel like overkill. Fire risk sits at the “moderate” end because the flash point gaps above 60°C, but it’s never a good idea to treat flammable liquids casually. Overexposure can provoke skin and respiratory irritation, so decent fume hoods and good airflow are a must, not just a line in the manual. Storage in cool, dry rooms, inside amber bottles, slows any run toward accidental polymerization; adding a stabilizer like MEHQ makes a real-world difference. I’ve watched old friends react with surprise at how easily a poorly capped container can turn gummy or worse, so procedures aren’t just for clipboard compliance, but real safety.
This monomer earns its place mostly in specialty coatings, adhesives, and advanced acrylic polymers designed for electronics, pharmaceutical carriers, and even additive manufacturing. I’ve seen project teams chase performance boosts in antistatic floors, dental devices, and optical films using this exact building block. Because it transforms into copolymers with higher gloss, improved water compatibility, or greater adhesion, it shows up everywhere from semiconductor clean rooms to construction adhesives. Product designers like the expanded playbook it offers—building in chemical resistance or electrical properties that pure methyl methacrylate can’t deliver. In the world outside the lab, that means less product failure, lower maintenance for infrastructure, and smarter interfaces in consumer electronics.
R&D teams circle this molecule for good reason. Scientists drive plenty of research into surface modifiers, next-gen drug delivery carriers, and smart hydrogels, all using variations of Ethylene Glycol Methyl Ether Methacrylate. The water solubility and reactive ether handle give chemists a launchpad—tuning behavior under light, temperature, or pH with just a few tweaks. I remember a collaborative program between a university and an adhesives giant, pouring months into optimizing tack and cure speed for electronics assembly. The compound’s flexibility, both chemical and operational, makes it a favorite for pushing the boundaries of what acrylic chemistry can handle. Each year, trade conferences and journals reveal new blends, smarter surfactants, and smarter approaches for controlled release or biocompatibility, all fed by curiosity and demand for better-performing materials.
You can’t ignore safety data, especially for ethers in the workplace. Studies show repeated skin contact risks dermatitis, and extended inhalation of vapors brings headaches or worse. Long-term exposure studies have flagged reproductive toxicity for related glycol ethers, and regulators keep tightening rules year by year. Ethylene Glycol Methyl Ether Methacrylate avoids some pitfalls of the shorter-chain ethers, but the parallels mean you still need strong controls and regular air monitoring. I remember a plant safety officer who’d survived more than his share of near misses—his war stories always started with attention lapses and ended with changes to operating procedures. MSDS documents make it plain: don’t treat this like a benign acrylic, and don’t shortcut protections for the sake of schedule. Good engineering controls and robust training back up those points, and laboratories that ignore them court trouble down the road.
Looking ahead, Ethylene Glycol Methyl Ether Methacrylate seems set for a broader role. Market forces pushing for greener, water-based materials lift demand for hydrophilic monomers, and this one delivers. As electronics get smaller, lighter, and more demanding, formulators turn to monomers that offer high performance in thin films or flexible circuits—exactly where this molecule fits. Driven by newer technologies—3D printing, bio-compatible polymers, high-performance coatings—the need for customizable acrylic building blocks seems likely to keep rising. Regulatory pressure will keep steering research toward less toxic alternatives or at least safer handling processes, and that’s not going to slow down anytime soon. Partner research between industry and academia promises to keep unlocking new functionalities—whether in smart wearables, bio-responsive materials, or more sustainable construction. With clever chemistry and commitment to safety, this monomer’s story is far from finished.
Look around, and you’ll spot plastics, coatings, adhesives, and paints shaping daily life. Most folks never think about the science underneath. Ethylene Glycol Methyl Ether Methacrylate, often shortened in labs as EGMEMA or EGME-MA, comes up often in the stories behind the scenes of modern materials. It’s a part of the methacrylate family, and its specialty involves helping certain materials do what they do best—sticking, stretching, resisting water, or forming tough, glossy surfaces.
In the paint shop or the plastics factory, people look for ingredients that won’t fight humidity. With some polymers and resins, you end up with cracking or warping as the weather changes. By blending in this methacrylate, companies produce coatings that stay put and keep their color. Small upgrades like this shift a product from “good enough” to “actually worth buying.” Take a walk through an auto showroom—those gleaming durable clearcoats rely on enhancements like this to keep cars looking new years down the line.
Not every plastic is meant to stay rigid. In adhesives and sealants, flexibility spells the difference between a one-off project and something that holds up for decades. By using EGME-MA, makers create glues and caulks that won’t grow brittle or crumble after a cold winter. Most crafters or builders might just reach for the tube, unaware of the long chemical journey that made their project possible.
Printing inks need to dry fast and stick well, especially in packaging plants churning out products by the minute. This specialty methacrylate helps inks grip glossy or flexible film surfaces—a big deal for things like chip bags or frozen food wrappers. Packaging waste gets all the headlines, but behind the scenes, the shift toward lighter, more recyclable wraps draws on innovations like this to keep food safe and print sharp.
It’s tempting to cheer for every chemical that makes things tougher, shinier, or longer-lasting. At the same time, it’s wise to remember what these substances can do outside the lab. Ethylene Glycol Methyl Ether Methacrylate, like many specialty chemicals, demands respect. Breathing in the vapors or splashing a bit during processing can lead to health risks for factory workers. I’ve seen up close how personal protective equipment and good ventilation turn from afterthoughts into lifelines on the shop floor.
Better training and stricter handling rules can keep workers safe, but there’s room for broader changes. Encouraging plant managers to invest in closed-loop systems, for example, traps fumes before they ever reach the staff. Regulators don’t need a new playbook—they just need spot checks and strong penalties for corners cut on safety. The point isn’t to scare anyone off chemistry. It’s to keep progress from leaving real people behind.
With every new use that appears—be it in sports gear, medical devices, or electronics—the push never stops for materials that last longer without sacrificing safety. I remember a visit to a local plastics plant, where new blends got stress tested every week. The crew up front knew the real-world demands: gear that bends, flexes, and bounces back again. They didn’t care for technical jargon, just results. EGME-MA is one of those tools that quietly transforms the daily things we count on. Yet the work doesn’t stop with what’s already on shelves. Responsible chemistry means looking for safer substitutes wherever possible and keeping a stubborn focus on both utility and safety.
Handling Ethylene Glycol Methyl Ether Methacrylate, for short, EGME methacrylate, shouldn’t feel like just another day at the lab. This stuff packs a punch. Its vapors can irritate eyes, skin, and lungs. It’s also absorbed easily through skin, so you risk more than just a rash if you touch it barehanded. In some workplaces, I’ve seen folks skip gloves for quick tasks, but that shortcut builds up risk. Breathing the vapors isn’t any better—long-term exposure has links to liver and kidney issues.
Gloves are the line of defense. Nitrile gloves block most of these solvents, and I never trust vinyl ones for this kind of job. Splash goggles or better yet, a full face shield, keeps your eyes safe. The one time I went without because my glasses fogged up, I wound up with watery, burning eyes for hours. Lab coats and closed-toe shoes aren’t for show; these protect you in case you knock over a bottle or splash your sleeves.
Ventilation can turn a risky task manageable. I once worked in a space without a fume hood; nobody could stand the headache and chemical smell after an hour. Even opening a window made a big difference. A chemical fume hood used right means you breathe easier and keep the workspace safer for everyone around. Staying below the occupational exposure limit makes a big difference for your long-term health.
It’s tempting to pour chemicals into any empty bottle, but proper labeling keeps coworkers out of trouble. I've seen harmful mix-ups from unlabeled flasks—one mistake can send someone to the emergency room. Safety Data Sheets stay nearby for a reason. It helps to review them regularly instead of waiting for trouble. Most of what you need to know—handling, storage, first aid—is right there. Skipping training isn't just risky, it puts everyone in the lab under pressure.
EGME methacrylate doesn’t just irritate. It’s flammable. Keep it away from open flames, hot plates, and electrical sparks. I’ve seen chemists pull out their phones right next to their workstations. Static and electronics don’t mix with flammable vapors. For storage, use safety cans and approved flammable storage lockers. Regular cleanups go a long way for spill prevention, and spill kits should always be within reach. Practicing drills—actually using the equipment you store—builds confidence.
PPE, good ventilation, correct labeling, and knowing emergency procedures truly protect you. Washing hands after handling, keeping eating and drinking out of work zones, and reporting problems right away set a safe culture. In my experience, team members who model these habits encourage everyone to step up. Prompt reporting of symptoms lets a supervisor deal with small issues before they grow. Investing in updated safety training and listening to frontline workers can solve problems others might miss.
Reading through the name “Ethylene Glycol Methyl Ether Methacrylate” sends most of us searching for a whiteboard. Each piece hints at the molecular makeup. The base, methacrylate, comes from methacrylic acid. This group brings a reactive vinyl group (C=C) stuck to a carboxylic acid backbone, which in this case gets switched into an ester. That “ester” part means someone has replaced the acid’s hydrogen with another carbon-based group. Enter “Ethylene Glycol Methyl Ether.”
This second part tells a chemist that a side-chain of two carbons, with an oxygen in between, attaches to a methyl group on one end. Back in school I learned to read these chemical names like puzzle clues: ethylene glycol gives two carbons and two oxygens, with “methyl ether” showing there’s a methyl (–CH3) at the tail. Link it together with methacrylic acid’s ester position, and you get a formula that grabs onto polymer chains and solves real-world problems in coatings and adhesives.
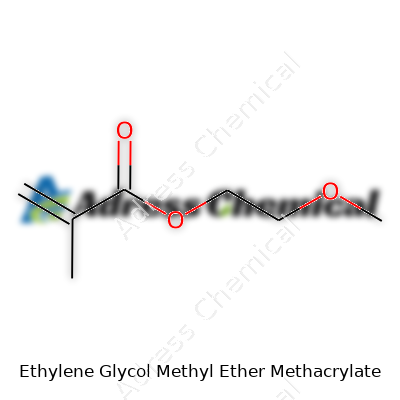
The structure runs as follows: the methacrylate group (CH2=C(CH3)COO–) latches onto the ethylene glycol methyl ether (CH2CH2OCH3). Put together, the complete formula reads C9H16O4. On paper, this shows up as:
CH2=C(CH3)COOCH2CH2OCH3
A little bit of chemistry goes a long way. That double bond on the methacrylate serves as the docking point for polymerization. The ester portion extends the chain, and the ethylene glycol methyl ether learns to mix with both water and organic solvents. That trick brings an edge, offering paints and coatings a tool for better flow, lower volatility, and friendlier behavior during application.
Digging past the formula, this molecule solves sticky problems every day. Imagine a factory shelling out coatings for metal pipes. Pure methacrylates might dry too fast or make films with little flexibility. Plugging in ethylene glycol methyl ether methacrylate changes things. Flexibility improves, surfaces get smoother, and the result stands up better to wear.
Safety tends to ride shotgun when talking chemistry. The methyl ether portion (sometimes labeled as a glycol ether) has caught scrutiny. Exposure to large amounts can affect health, so keeping the workplace ventilated and wearing gloves isn’t just a suggestion. Statistics from industrial hygiene studies show lowered exposure can prevent issues like skin irritation or, with higher exposure, more serious health worries. A smart shop manages air quality, and robust labeling rules keep incidents low.
One issue that always crops up: environmental persistence. Glycol ethers can slip through wastewater treatment if facilities don’t use specific methods. Regulators ask manufacturers to investigate greener tweaks. Swapping in similar monomers with shorter half-lives or easier breakdown pathways matches up with ongoing efforts to “green” the whole product lifecycle. The push to advance ‘biobased’ alternatives drives real change, but performance trade-offs often drag behind.
The bottom line—ethylene glycol methyl ether methacrylate gives chemists and manufacturers a lever for better products, but it doesn’t come free of responsibility. Reading the structure isn’t just textbook trivia; it leads to real decisions about safety, performance, and environmental cost. If the industry keeps experimenting and the community stays vigilant, smarter solutions will follow.
Ethylene Glycol Methyl Ether Methacrylate. The name alone is enough to make most folks pause before grabbing a container. This liquid, common in a variety of specialty coatings, adhesives, and polymer production, doesn’t ask for special treatment for kicks—it requires it for everyone’s safety and peace of mind. My background in chemical handling taught me early that with these kinds of chemicals, small mistakes can lead to big regrets.
Let’s clear out the fog: This substance isn’t just another shelf-filler. It carries health risks—skin and eye irritation, respiratory troubles, and other complications if you let it hang around in open containers or in a warm room. Workers with long-term exposure have faced serious consequences. Tossing an extra filter mask into the kit isn’t enough—real safety starts with how you stash the stuff.
In warehouses or small shop corners, flashy hazard stickers can lull people into thinking everything’s covered. Good storage begins with a space that puts a lid on three big threats: heat, moisture, and light. From my own experience, tucking these chemicals away in a dark, dry storeroom, with a steady, cool temperature, means you sleep easier at night. I’ve watched heat and direct sunlight break seals or warp plastic before; letting that happen opens the door to leaks and ruined product. At forty degrees Celsius, this stuff starts to carry a vapor pressure that’ll push against even strong containers. Even at half that temperature, things can still go sideways.
Nothing beats a proper metal drum or HDPE (high-density polyethylene) jerry can. Keeping the cap tight, but not stressing seals to the cracking point, gives a buffer against accidental spills. I learned not to trust container placement to luck; keeping them on spill pallets or metal shelving takes just a few minutes but saves a lot of cleanup—and sometimes, a call to emergency services.
Stale, closed-in storage spaces build up vapors, especially in warm weather. One summer, I opened an old closet in a poorly ventilated corner and got a face full of fumes—lesson learned. Airflow keeps vapors down and helps people stay safe. Flammable materials, acids, or oxidizers should always sit far away from any container of this compound. Mixing the wrong neighbors, even by accident, turns a dull day into a dangerous situation.
No amount of fancy shelving helps if nobody knows the protocols. Everyone with access should know how to deal with leaks, what happens if a container cracks, and how to put on the right gloves, goggles, and aprons. I’ve watched even experienced crew skip steps when they’re in a hurry or assume someone else “probably checked everything.” Prioritizing refreshers, even if some grumble, keeps the whole operation running smoother.
Short-term savings from buying cheaper containers or packing chemicals too close often come back as big bills—damage to inventory, health claims, or fines from inspectors who take one sniff of the air and spot a dozen mistakes. Tracking your inventory and noting expiry dates can stop forgotten containers from turning into a bigger problem later on.
Solutions don’t need to break the bank. Setting up a locked cabinet with ventilation holes, assembling a spill kit with clay absorbent and neutralizing powder, or holding brief drills, each step makes a real difference. People remember clear rules and hands-on practice far better than a binder full of guidelines stuck in a drawer.
Taking extra care with Ethylene Glycol Methyl Ether Methacrylate isn’t just following regulations—it's about respecting the folks handling it and the environment we all share.
Most folks rarely think about chemicals like Ethylene Glycol Methyl Ether Methacrylate (EGMEMMA). The name itself sounds like a tongue twister or a complicated experiment tucked away in a distant laboratory. But the reality: this compound shows up in paints, coatings, and adhesives. Some workers spend their days breathing its fumes or getting it on their hands. The question is, what does prolonged contact with this chemical mean for health?
Years in old auto shops and paint rooms tell the story. Colleagues occasionally came home with foggy heads or dried-out skin after a shift near acrylics or resins. Many never knew exactly which chemical caused what, but evidence has caught up. Inhalation or skin absorption of EGMEMMA pushes real risks onto the table.
Research points to headaches, nausea, and even confusion. Some folks deal with persistent dizziness or trouble with their memory after regular exposure. There’s another side to it: eye and skin irritation can show up almost immediately after a splash or spill. I’ve witnessed a friend get persistent eczema from solvents. No good ever comes from ignoring repeated rashes or chemical burns, especially if the culprit is something as reactive as EGMEMMA.
A bigger worry floats beneath the skin. Studies link this compound to damage in the kidneys and the blood system. This means workers might not feel the toll until years later, once organ function starts dropping or lab results catch unexplained anemia. EGMEMMA can travel through the bloodstream after absorption, collecting in vital organs, and some byproducts react poorly over time. Chronic exposure often gets overlooked in crowded workplaces, even though science screams for regular medical checks.
Perhaps the most sobering fact: certain glycol ethers harm fertility or fetal development. Animal studies connect these chemicals to birth defects and miscarriages. Some countries have gone as far as to restrict use in various consumer goods for this reason. Workers often juggle jobs and family life. Few want to imagine their tools on the clock creating risks at home, especially for those planning to raise children.
Safety culture starts at the jobsite. I’ve seen simple investments in gloves, goggles, and exhaust fans save emergencies in shops with chronic ventilation problems. Training matters even more. Without clear labeling and instruction, old-timers downplay new risks, and rookies don’t know what to avoid. Employers can choose safer substitutes or set up strict controls. Aware teams spot spills, keep skin covered, and never eat lunch at a chemical workstation.
Regular monitoring ensures no one absorbs levels that slowly poison their body. Medical checkups including blood work—especially in manufacturing—should be routine, not a luxury. Workers should pressure managers for transparency. A few honest conversations serve the whole team better than hoping for the best.
Many people trust “someone else is watching out for us” in tough industries. But facts around things like EGMEMMA push us to question that faith. It takes vigilance, not wishful thinking, to protect health. Whether it’s a shop floor, lab, or garage, the story stays the same. People demand honest information, practical solutions, and a willingness to act early—because no job is worth risking kidneys, memory, or a family’s future.
| Names | |
| Preferred IUPAC name | 2-methoxy-1-methylethyl 2-methylprop-2-enoate |
| Other names |
2-Methoxyethyl methacrylate EGMEMA Methacrylic acid 2-methoxyethyl ester 2-Methoxyethoxy methyl methacrylate MEMA |
| Pronunciation | /ˌɛθ.ɪˌliːn ɡlaɪˈkɒl ˈmɛθ.əl ˈiːθ.ər ˌmeθ.əˈkræ.leɪt/ |
| Identifiers | |
| CAS Number | 110-98-5 |
| Beilstein Reference | 1322076 |
| ChEBI | CHEBI:87199 |
| ChEMBL | CHEMBL2087446 |
| ChemSpider | 21719679 |
| DrugBank | DB14645 |
| ECHA InfoCard | 03b7b09d-2b43-4ead-b86a-b028c84a92b0 |
| EC Number | 203-473-3 |
| Gmelin Reference | 1095456 |
| KEGG | C18506 |
| MeSH | C04.557.450.550.700.500 |
| PubChem CID | 13377 |
| RTECS number | KM1925000 |
| UNII | R7NP817JZD |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID0050788 |
| Properties | |
| Chemical formula | C7H12O3 |
| Molar mass | 190.22 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Sweet odor |
| Density | 1.039 g/cm3 |
| Solubility in water | Miscible |
| log P | -0.2 |
| Vapor pressure | 0.38 mmHg (20°C) |
| Acidity (pKa) | pKa = 15.5 |
| Magnetic susceptibility (χ) | -7.59×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.424 |
| Viscosity | 1.238 mPa·s (25 °C) |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -509.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3547 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Suspected of damaging fertility or the unborn child. |
| Precautionary statements | P210, P261, P280, P303+P361+P353, P305+P351+P338, P370+P378, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-2-2-W |
| Flash point | 84°C |
| Autoignition temperature | 230 °C (446 °F; 503 K) |
| Lethal dose or concentration | LD50 (oral, rat): 1850 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,068 mg/kg |
| NIOSH | RN 0185 |
| REL (Recommended) | 0.1 ppm |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Methyl methacrylate Ethylene glycol methyl ether Ethylene glycol dimethacrylate Methacrylic acid Ethylene glycol monomethyl ether acetate |