Ethylene Glycol Methyl Ether Acetate, often called EGMEA, started making a difference in the mid-20th century. The chemical industry was picking up steam, and chemists looked for better ways to dissolve tough resins and coatings. Paint, electronics, ink—these areas depend on solvents that evaporate predictably and can handle both polar and non-polar ingredients. In the early days, workers handled raw chemicals directly, and after some pretty rough lessons, regulations followed. Each decade brought stricter workplace standards. Personal experience tells me: anytime a product sticks around for decades and adapts to tougher rules, it’s doing something important. EGMEA didn’t just survive regulatory scrutiny. It changed to fit the industry’s needs, finding a place in both factories and labs from big cities to small workshops.
You can spot this solvent on a label by the names 2-methoxyethyl acetate, methyl cellosolve acetate, or its CAS number, 110-49-6. Its liquid, colorless form comes with a sweet, slightly fruity smell that hints at its chemical backbone. Most folks see it as just a means to mix other ingredients, but there’s more going on. This acetate isn’t about being flashy. It gets the job done where other solvents fall short, especially if you’re trying to break down something stubborn or keep electronics free of residue. In paints and coatings, it lets pigments flow evenly and extends drying time so surfaces don’t bubble or crack. The electronics sector counts on it when making chips—those shiny wafers have features too tiny for dust, and residue can ruin an entire batch. In my own work, I’ve been in labs where making a mistake with a solvent meant lost time and ruined dollars, not just inconvenience.
EGMEA doesn’t try to be mysterious. It boils at around 145°C and freezes at -64°C. The stuff is lighter than water, with a density of about 0.965 g/cm³, so it floats if spilled in a puddle. It mixes well with other solvents and even water, which gives it a big advantage over heavier, oil-based solvents. Its vapor pressure—measured at about 3.7 mmHg at 20°C—means it hangs around longer than acetone but dries faster than some glycol ethers. The molecule is a blend between volatile and stable, cutting through grease and resin without leaving behind oily traces. These properties let factories choose it for jobs needing both cleaning power and finesse, from photoresist stripping to final cleaning in medical device production.
Most manufacturers offer technical data sheets with exact purity figures, typically claiming at least 99% pure EGMEA. Water content stays below 0.05% in top-grade batches. Labels list hazard warnings clearly: flammable, irritant, and always a reminder about reproductive risks. I’ve seen containers with QR codes that pull up the full material safety data sheet (MSDS) on your phone, which shows just how vital clear information has become. Barrels and bottles come stamped with batch numbers, production dates, and even recommended storage temperatures. Nothing in the chain gets left to chance, especially after a few highly-read news stories about worker exposure led to stricter oversight and more transparent labeling.
Making EGMEA doesn’t sound flashy, but it involves serious chemical control. Production starts with ethylene oxide and methanol in a process that forms ethylene glycol methyl ether. Add acetic acid (or acetic anhydride) and run the reaction under a controlled temperature—you get the acetate ester. The efficiency of this reaction makes large-scale production possible, but a small change in conditions can create impurities or dampen yield. Technicians keep a close eye on temperatures and pressure, and automated sensors have eased some of the headaches. From personal experience, walking through a chemical plant near the reactor units is noisy, smelly, and always just a little tense. These aren’t settings for careless mistakes. Every leak or spike in temperature often means a rush to safety showers and an investigation afterward.
EGMEA pops up as both a starting material and a solvent for other reactions, and it can undergo hydrolysis in acidic or alkaline environments, breaking down to methoxyethanol and acetic acid. Fire up a reaction with a strong base, and you might see cleavage products that can irritate the skin and lungs, highlighting the need for careful oversight wherever this solvent is used. I remember a university lab project where an improper neutralization left behind enough EGMEA fumes to set off alarms. Later, we learned the importance of vent hoods and up-to-date chemical inventories. The take-home lesson: solvents with “ether” in their names often hide surprising reactivity, requiring respect and planning.
In catalogs and on barrels, egmea goes by a few names—2-Methoxyethyl acetate, Cellosolve acetate, and methyl glycol acetate, among others. Some suppliers call it EGMEA outright. The wide range of aliases sometimes makes ordering supplies tricky, especially for small-scale users who may find themselves with the right chemical but under the wrong label. Over the years, I’ve heard enough mix-ups to double-check every label, especially when a delayed shipment can cost a lab days of downtime.
No one can work safely with EGMEA by luck alone. The solvent’s reputation for reproductive toxicity led to its restriction in many settings, especially those with pregnant workers. Both OSHA (in the US) and the European Union set tight occupational exposure limits—typically well below 1 ppm for the long term. Good personal protective equipment (PPE) includes nitrile gloves, eye protection, and fume hoods or well-ventilated rooms. Industrial sites enforce spill kits on every floor and have detailed response plans posted near workstations. From what I’ve seen, regular safety drills and surprise inspections push compliance. The price for sloppiness can be high—skin exposure burns, inhalation leads to drowsiness, and over time, the risk to fertility is well-documented. Never once have I regretted taking an extra ten seconds to check a glove for pinholes or double-check a vent.
Painters, ink makers, and semiconductor plants rely on EGMEA for its unique knack for dissolving complex mixtures and its controlled evaporation rate. Coating production jumps in efficiency when the right solvent keeps pigments suspended just long enough for uniform drying. The electronics world won’t trust anything that leaves residues behind—circuit lines on chips are measured in nanometers, and any leftover film spells disaster. Screen printing, photoresist stripping, and specialty adhesives rely on EGMEA, too. I’ve watched a broken supply chain for this solvent throw entire production lines into chaos, with engineers looking desperately for substitutes that just couldn’t get the same finish and performance.
Research teams still study the limits and alternatives to EGMEA, usually aiming to reduce environmental and health impacts. Green chemistry labs are busy developing bio-based solvents that mimic its properties without the same toxicity. Universities run small-batch synthesis trials, searching for catalysts that would convert simple alcohols into useful acetates at room temperature. Journals report on recycling projects where spent solvent gets cleaned, separated, and re-used instead of heading straight for incinerators. Years ago, I saw a university pilot project where reused EGMEA ran through a membrane filtration system, stretching every liter and cutting disposal fees. These small steps matter in an industry where every solvent drum often equals hundreds of kilograms of chemical waste.
Animal and human research leaves little doubt about EGMEA’s dangers—yes, it’s absorbed through skin and lungs, then distributed to organs and even developing embryos. Symptoms swing from simple irritation to blood and kidney issues after chronic exposure. Several studies, including those referenced by the US EPA and European Chemicals Agency, linked prolonged contact with birth defects and changes in blood chemistry. Unions and advocacy groups bring these facts into contract negotiations, pushing for safer substitutes and routine air monitoring. Small improvements in detection—like low-cost colorimetric badges and wearable gas sensors—help spot leaks before people get hurt.
Regulation keeps tightening, and companies look for ways to swap out EGMEA for friendlier alternatives without losing the performance that makes it valuable. Digital manufacturing demands even higher purity and consistency from solvents, so any drop in quality shows up fast. Some innovators focus on closed-loop systems where nothing escapes into the air, recovering and cleaning solvents for reuse. Others unlock bio-based substitutes from renewable feedstocks that, with luck, won’t carry the same toxic risks. The future for EGMEA may shrink in workplaces but its story shows how chemistry chases balance: performance, cost, and safety—rarely do all three grow together. Yet just as in the early days, every shift in the industry brings another round of learning and adaptation.
Most folks don’t spot ethylene glycol methyl ether acetate on the shelves at home improvement stores. But peek behind the labels of many commercial paint cans, specialty coatings, and high-grade inks, and there it is — hiding in plain sight, doing some heavy lifting. This solvent carries enough punch to dissolve tough resins but still behaves well enough for manufacturers who need reliable results. My own days in a print shop made it clear: without this chemical, inks just didn’t spread, set, or look right on a proper press run.
Electronics don’t get much credit for their shiny cases and crisp PCB lines. Ethylene glycol methyl ether acetate steps in here too. Big names in semiconductor manufacturing lean on it to stop photoresists from clumping or running during the chipmaking process. In cleanroom jobs, the tiniest mistake leads to a pile of rejected parts, so a solvent that cleans without streaking or residue quickly earns its spot on the shop list.
Automotive plants face a similar story. Modern clear coats—the kinds that make new cars gleam—need careful mixing and precise drying times. Too slow, and assembly lines back up, costing everyone money. Too quick, and the finish clouds or fails. A chemical like this one helps tune the process. From spray booths to detailing bays, its balancing skills matter.
Nobody wants a good solvent at the cost of worker health. The trouble is, ethylene glycol methyl ether acetate carries risks if inhaled or absorbed through the skin. I’ve toured factories where safety folks keep a close eye on ventilation, masks, and gloves. Stories in the industry circle around headaches and fatigue—those unmistakable signs that the air’s not as clean as it needs to be. Safety data isn't just paperwork; OSHA marks this compound as hazardous in certain levels over time, linking overexposure to issues with the blood, kidney, and even the nervous system.
Companies have to weigh performance against people’s well-being. Sometimes it means doing air quality tests more often, swapping out this chemical where greener options work, or rotating jobs so nobody gets stuck in the same area all shift. Even on small teams, those rules matter. An ink-spattered apron doesn’t look out of place to outsiders, but the right PPE—proper gloves, goggles, serious exhaust fans—can mean the difference between a healthy worker and someone feeling rough halfway through the week.
With new laws and public pressure, industries want chemicals that don’t just work—they should break down without long-term messes and keep everyone safer. Water-based paints have gained ground, but time and again, specialty coatings and electronics circles return to ethylene glycol methyl ether acetate for consistency. It’s a process: trialing alternatives, tweaking formulas, testing again. Research groups and eco-startups now turn out biobased or less toxic alternatives that handle many tasks nearly as well.
It’s not about scrapping everything overnight. Each step forward—safer storage, closed-loop systems that catch fugitive vapors, real investment in substitutions—chips away at the old risks. What sticks out the most for me: people pay closer attention now, and they talk openly. If a paint or a chip can meet the mark with less exposure for workers and the environment, fewer folks want to stick to the way things used to be done.
Working around chemicals like Ethylene Glycol Methyl Ether Acetate can change the way you think about safety on the job. Most people don’t run into this solvent unless they’re dealing with paints, inks, or electronics work. I remember the first time I opened a drum of this stuff, the strong, sharp smell hit me, and it made me step back and rethink whether a pair of cheap gloves and an open window would cut it. Turns out, it wouldn’t.
Skin absorbs certain chemicals much faster than we think. This ether acetate goes right through, which can lead to headaches, nausea, and worse if you deal with it day after day. Regular nitrile gloves do the job. I’ve tried using reused household rubber gloves in a pinch, and my hands got sticky and irritated. Chemical-resistant gloves, goggles, and a face shield do more than check off a box—they stop the solvent from finding its way into your body.
A stuffy room makes this chemical even riskier, especially because the vapors rise quickly without you noticing. I’ve worked in small shops where the only air movement comes from an ancient fan in the corner. It’s not enough. Large exhaust hoods or local exhaust fans, right over the workspace, pull the vapors out before they fill your lungs. OSHA’s limits (25 ppm over 8 hours) aren’t set for nothing—spending time over those levels will throw your health off balance.
Once, someone dropped a partly open can and watched the liquid snake across the floor, soaking into concrete and sending those unmistakable fumes right into everyone’s faces. We learned to keep spill kits nearby—absorbent pads, not just paper towels. Fire is another worry. Vapors from this ether catch fire fast. It belongs in flammable storage cabinets, away from sparks and hot gear. I’ve seen supervisors leave open containers out, inviting trouble. Tighten those lids, mark the cans, and don’t mix containers.
Getting regular checks might seem like overkill, but chronic exposure sneaks up. Some folks get nerve problems or liver changes, long after the fact. Blood and urine tests, even if you feel fine, can catch issues before they drag you down. I’ve lost coworkers to illnesses that crept in after years on the line. Take the masks, gloves, and tests seriously. If anything starts to feel wrong—dizziness, fatigue, or confusion—stop and say something. Your body gives signals for a reason.
Training goes beyond pointing at a wall chart and signing a logbook. I’ve seen the best safety culture come out of teams who talk through every step, double-check each other’s gear, and report problems without worrying about getting blamed. Emergency showers and eye wash stations should be easy to reach, not buried behind storage boxes. People remember small things—washing hands before lunch, never carrying open containers, tying back loose clothing. These habits keep the work steady and safe, day in and day out.
Ethylene Glycol Methyl Ether Acetate shows up in coatings, inks, and electronic parts. It works as a solvent, moving things along until folks finish the job and move products out the door. What often gets overlooked is how quickly one slip during storage or handling turns a useful chemical into a health problem or a fire hazard. Chemicals like this one do more than sit quietly on a shelf; they demand respect and a steady hand.
This solvent carries a low flash point, which means it can catch fire more easily than water boils on a stovetop. A small spark, static charge, or hot machine close by can light up a spill or vapor cloud before anyone has the chance to react. Having worked close to paint shops, I saw workers stack solvents near space heaters or under makeshift fans. Accidents came less from carelessness and more from letting convenience overrule caution. Fire extinguishers are a last line of defense, not a license to ignore risk. Drums and tanks made from the right metal, sealed tightly, and grounded against static stand between normal days and chaos.
Young techs often underestimate what breathing in these solvents does to their system. Shortness of breath, headaches, or an irritated throat seem minor, but long-term exposure builds up. Without fans to pull fumes away, or masks that really fit, these troubles won’t fade by the next shift. Some companies aim for cost savings by skipping proper venting or simply propping open windows. That sort of shortcut ends with sick workers and half a day spent filing reports for a visit to the clinic. Facility managers should treat real ventilation and personal protection like bill payments—expected, non-negotiable, and checked regularly.
I’ve stood in shops where “spill kits” meant a broom, a mop, and the hope that rain would come and wash away the rest. Ethylene Glycol Methyl Ether Acetate runs quickly and seeps into cracks, making even a small leak tough to control. Absorbent pads rated for solvents, quick access to gloves and goggles, and clear lines between clean and dirty zones matter much more than laminated instructions pinned to a wall. Spill response training ought to rise to the same level as fire drills—practiced often, adjusted for new people, and never left to guesswork.
Disposing of leftover solvent or contaminated gear takes more than pouring it down a drain or into a dumpster. I’ve watched crews let barrels sit in the sun, corrosion eating at the seams, only to scramble during a surprise inspection. Waste storage rules exist for a reason. Locking up used containers in well-marked, ventilated sheds shields both workers and the soil outside. It also keeps neighborhoods safe and companies out of hot water with local agencies.
Sheltering toxic solvents behind locked doors, with working alarms and emergency wash stations nearby, costs less than medical bills, shutdowns, or lawsuits. Training every new hand who walks onto the floor turns good intentions into muscle memory. Those extra minutes spent logging inventory, checking seals, and reviewing safety sheets make the difference between a long career and an early headline. There’s no room to cut corners or gamble with safety—too much hinges on small choices made every single shift.
Factories use all sorts of chemicals to keep the world running, and Ethylene Glycol Methyl Ether Acetate (EGMEA) often turns up in paints, inks, and electronics production. Some folks work around EGMEA every shift and rarely stop to ask if the risk feels worth the paycheck. Although that strong, sweet smell may hint at danger, it’s the things you can't see that often cause the most harm. Breathing in EGMEA, even at levels below the immediate irritation threshold, takes a toll on the body with steady, regular exposure. Skin and eyes might sting, but the problems run deeper than a little discomfort.
I remember talking to a lab tech who watched three colleagues develop headaches, fatigue, and trouble focusing after swapping to new solvents. Those weren't mild symptoms for them; turns out the company switched to a cheaper cleaning solvent that listed EGMEA on the safety data sheet. Sensing something wasn’t right, they pressed for air monitoring. Results didn't help—air levels checked in at what the rules call “acceptable,” but workers still showed blood abnormalities at their annual checkups.
Doctors make it clear: EGMEA can attack red blood cell production and even harm bone marrow. In animal studies, high doses cause birth defects and reproductive harm. Nobody needs a degree to know messing with blood or reproduction spells trouble for everyone, especially folks of childbearing age. Masking the floor with fans or relying on old exhaust hoods isn’t much comfort if you’ve seen how slowly chronic symptoms appear—and how tough they are to reverse once they settle in.
Consider how easily liquids soak through your skin, especially solvents like EGMEA. Gloves labeled for “chemical resistance” sometimes leak sooner than their packaging promises, and busy shifts leave little time to swap out gear after a splash. Inhaling vapors builds up in the blood, and that steady trickle over months plants the seed for lasting toxicity. I once watched a co-worker ignore handwashing advice, figuring soap was good enough. That changed fast—he ended up with persistent dermatitis and constant low-grade sickness until he switched departments.
Rules exist, but the paperwork often lags behind the science. The Occupational Safety and Health Administration sets Permissible Exposure Limits, yet research continues to show harm at levels lower than these guidelines. Many countries have adopted stricter restrictions or banned the stuff outright in certain industries. Companies serious about safety have replaced EGMEA with substitutes that carry less long-term risk, but transition costs slow things down. It helps to remember—health care and lost work pile up a bigger bill over time than most solvent swaps.
Personal protective equipment plays a part, but real change comes from pulling EGMEA out of the process altogether. I’ve seen shop floors thrive on water-based products after ditching this chemical, air cleaner, and absentee numbers dropping. Managers grumbled about upfront costs but quickly forgot when insurance rates stayed low and staff turnover improved. Signs, training, and oversight only do so much. Removing the hazard beats managing it, every single time.
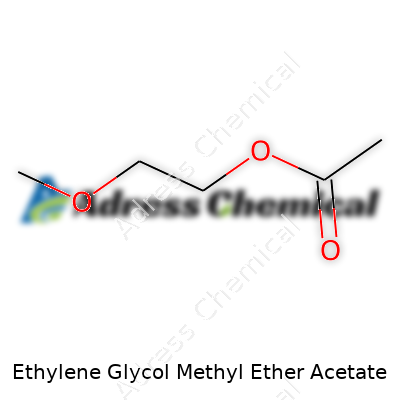
Ethylene Glycol Methyl Ether Acetate – often shortened to EGMEA or 2-Methoxyethyl Acetate – carries the chemical formula C5H10O3. Looking it up in a chemical database, you’ll see it listed with the CAS number 110-49-6. These aren’t just numbers and letters for a textbook. In practice, they told me exactly what I was working with back in my early lab days, especially during solvent selection for a stubborn polymer project. Clarity around chemical identity helps everyone from researchers to factory workers keep safety and quality in focus.
I remember the first time I stood in a coatings facility, watching vats of raw solvents stream into mixing tanks. EGMEA stood out as a favored ingredient for certain inks and coatings – not just because it performs well, but because it dissolves both polar and non-polar substances. That’s no small feat. You find small details like this matter on a busy line, where a batch failure costs real time and cash. Workers on the floor, myself included, didn’t want just a chemical that worked. We wanted one that didn’t introduce more hazards than the job already held.
Chemists and technicians should keep an eye on exposure. EGMEA can be absorbed through the skin, and inhalation sneaks up on you when ventilation falters. The stuff isn’t as notorious as some benzene derivatives, but regulatory agencies haven’t given it a clean bill either. I saw a few colleagues brush off gloves and take short-cuts, and over time, someone would always end up in the nurse’s station. Awareness and training will always beat wishful thinking for workplace safety.
Understanding that EGMEA stands for C5H10O3 and is tracked as CAS 110-49-6 arms professionals with the details needed to pull up technical sheets, hazard data, and rules put down by groups like OSHA or REACH. It’s not uncommon to see well-meaning folks treat these chemicals as interchangeable; experience taught me that’s a recipe for trouble. Using the wrong solvent, even a close cousin, can damage materials, alter drying times, and in some cases, spark unplanned chemical reactions. The numbers push you toward the right info, quick answers, and safer handling.
Engineering controls, like upgrading ventilation or using closed-loop systems, make a concrete difference. I’ve seen places turn to less hazardous substitutes, though that’s not always an open-and-shut option. In many small businesses, change happens slower because costs matter and production targets loom large. The best gains in safety and performance come from giving frontline folks more say and more training – not just a stack of protocols taped to the wall. From my time on the line, a ten-minute refresher and clear labels at point-of-use did more to prevent accidents than any policy manual ever did.
The chemical world doesn’t revolve around formulas and CAS numbers alone, but that’s where careful work starts. Each identifier gets you a step closer to smarter, safer handling and better results – in the lab, on the factory floor, or wherever chemicals mix with daily life.
| Names | |
| Preferred IUPAC name | 2-methoxyethyl acetate |
| Other names |
2-Methoxyethyl acetate Methyl Cellosolve Acetate EGMEA Methylglycol acetate Methyl oxitol acetate |
| Pronunciation | /ˈɛθ.ɨˌliːn ˈɡlaɪ.kɒl ˈmɛθ.əl ˈɛθ.ər əˈsiː.teɪt/ |
| Identifiers | |
| CAS Number | 110-49-6 |
| 3D model (JSmol) | `JSmol.loadInline("data/mol=WGoYJCDYKDGHDA-UHFFFAOYSA-N;format=mol")` |
| Beilstein Reference | 1208733 |
| ChEBI | CHEBI:31537 |
| ChEMBL | CHEMBL154054 |
| ChemSpider | 6966 |
| DrugBank | DB14019 |
| ECHA InfoCard | ECHA InfoCard: 03c724908cc9-40b2-b33b-ce1d8840baa0 |
| EC Number | 203-603-9 |
| Gmelin Reference | Gmelin Reference: 2092 |
| KEGG | C19697 |
| MeSH | D051439 |
| PubChem CID | 8646 |
| RTECS number | KO2450000 |
| UNII | 4M89B9UO8M |
| UN number | UN1171 |
| CompTox Dashboard (EPA) | DTXSID5020698 |
| Properties | |
| Chemical formula | C5H10O3 |
| Molar mass | 132.16 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | mild pleasant |
| Density | 0.965 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.36 |
| Vapor pressure | 2.5 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 16.3 |
| Magnetic susceptibility (χ) | -48.6×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.399 |
| Viscosity | 1.2 mPa·s (at 25°C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 308.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −616.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3500.7 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H312, H332, H360 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P260, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P312, P314, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 42°C (closed cup) |
| Autoignition temperature | 331 °C |
| Explosive limits | 1.5–10% |
| Lethal dose or concentration | LD50 oral rat 8532 mg/kg |
| LD50 (median dose) | 1,300 mg/kg (rat, oral) |
| NIOSH | K012 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Ethylene Glycol Methyl Ether Acetate: 25 ppm (120 mg/m³) |
| REL (Recommended) | REL (Recommended Exposure Limit) for Ethylene Glycol Methyl Ether Acetate is "0.5 ppm (2 mg/m³) TWA". |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol methyl ether Ethylene glycol Ethylene glycol diacetate Diethylene glycol methyl ether acetate |