Ethylene glycol diacetate did not arrive as a blockbuster during its early years in the chemical industry, but ingenuity often finds value where others see ordinary solvents. Originally, it appeared after chemists in the mid-twentieth century explored alternatives to aromatic solvents, which posed more health and environmental challenges. Large-scale synthesis took off in regions with rapidly expanding textile and coating industries, especially as demand for less hazardous and more stable solvents outpaced what traditional acetates could offer. Even though its story shares space with many molecules born from the never-ending search for better solvents, ethylene glycol diacetate gained respect as specialists found that its low volatility and mild odor helped in work environments where exposure limits mattered. Over time, economies with robust paint, ink, and agricultural processing industries recognized its value, especially where government regulation around solvents tightened.
Anyone handling solvents knows the frustration of choosing between efficiency and safety. Ethylene glycol diacetate leans into both. It brings relatively high solvency for cellulose derivatives, resins, and some plastics but doesn’t overwhelm the nose or evaporate too quickly. Manufacturers ship it mainly as a clear liquid, often packed in drums or tanks lined to resist acetic acid traces. Often called EGDA, it sits on safety datasheets next to other glycol esters, but head-to-head, it stays less pungent than some heavy-duty choices, making life around storage tanks and mixing rooms less miserable. Painters, print shops, and coating professionals appreciate a slow-evaporating solvent on humid or windy days since it helps coatings set up smoothly.
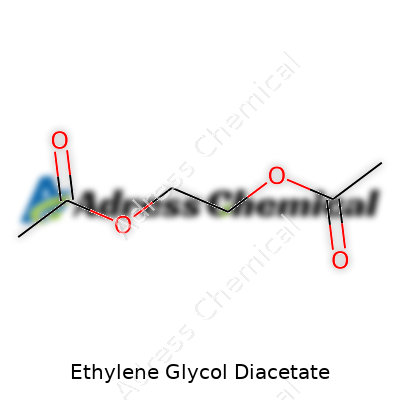
Ethylene glycol diacetate coats glassware in laboratories as a colorless liquid, mixing well with most organic solvents, but leaves water behind because of its hydrophobic tail. Bumping up the temperature shows its boiling point clocks in near 285°C, a far cry from lighter, more flammable acetates. This matters in formulation rooms while handling heated reactions or when setting up distillation columns. Density hovers around 1.12 grams per cubic centimeter, which keeps it apart from less robust acetates on the mixing line. Handling gets easier since vapor pressure stays quite low at room temperature. Its molecular formula, C6H10O4, shows the backbone of a diester, which brings stability but also means spills linger unless cleaned properly.
Drums or totes labeled for ethylene glycol diacetate display hazard pictograms for flammable liquids and mild skin irritants, although its risk ranks lower compared to classic volatile organic compounds. Purity grades matter in paint and resin work — technical grade usually climbs above 98%, with acidity checked by titration and color kept below 10 on the APHA scale. Most suppliers include CAS registry number 111-55-7, along with clear statements about flash point and recommended storage temperature, often printed in bold for warehouse teams. English, Chinese, or German labels circle around much the same details, since regulators around the world expect harmonized standards for industrial chemicals these days.
Production lines for ethylene glycol diacetate follow a clear path: ethylene glycol reacts with acetic anhydride, with acetic acid as a potential byproduct. Heating kicks off the esterification, usually in the presence of an acidic catalyst like p-toluenesulfonic acid. Purification splits off unreacted feedstock by distillation, passing the crude product over columns to hold back color bodies and trace acids. Technicians always monitor water content and acidity since stray moisture or leftover catalyst spoils both shelf life and performance for sensitive applications. Large-scale plants run batch or continuous reactors depending on customer demand, always banking on careful temperature control to avoid product loss from side reactions. In university labs and pilot plants, glassware only rarely sees this molecule, but industrial lines routinely turn out thousands of tons every year.
Chemists know this ester resists hydrolysis under moderate conditions, holding up better than many acetates exposed to moisture. In basic solutions, saponification produces ethylene glycol and acetic acid, an outcome rarely welcomed in coatings unless specifically required for degradation. On the synthetic side, ethylene glycol diacetate often appears as an intermediate, channeling the glycol core toward other esters or used as a non-participating solvent in acylation reactions. With strong acids, especially at elevated temperatures, hydrolysis accelerates, so maintenance teams and engineers watch for acid leaks near storage or process lines. In a reaction toolkit, its relatively unreactive nature makes it more of a carrier than a star participant, but that reliability shows up in processes needing controlled environments.
You’ll see ethylene glycol diacetate listed on supplier websites and technical catalogs under a handful of names: 1,2-Ethanediol diacetate, Glycol diacetate, EGDA, Diacetoxyethane, and Ethane-1,2-diyl diacetate. Multinational producers often clear up confusion by including the CAS number, especially in deals with global partners. Trade names sometimes appear in specialty catalogues, especially in coatings or ink chemistry circles, but smaller buyers usually stick with the simplest variant — glycol diacetate — to avoid mix-ups.
Industrial users treat this solvent with respect, based on inhalation limits published by organizations like OSHA and ACGIH. Even though its vapor doesn’t carry the punch of high-sensitivity irritants, contact can bring on headaches or mild respiratory irritation, especially in confined or poorly ventilated workspaces. Operators wear splash goggles and gloves on mixing decks, and exhaust systems run year-round for bulk storage rooms. Waste handling experts warn against dumping large volumes down drains, since breakdown products can upset wastewater plants. European regulations and the EPA set permissible exposure limits, and most plants keep documentation close at hand during inspections. Spill drills and cleanup stations keep teams ready in case containers leak or rupture, especially since insurance and supply contracts often hinge on adherence to these procedures.
Paint and coating rooms, printing presses, and agrochemical mixing plants all keep ethylene glycol diacetate on hand for its slow evaporation and gentle dissolving power. Printing ink manufacturers face pressure as solvent emission laws tighten; glycol diacetate fits the bill when lighter acetates evaporate too quickly or smell too sharp. Coatings derived from cellulose acetate or PVC-based resins turn to glycol diacetate for better flow, fewer bubbles, and less tack during drying. Sometimes, even pharmaceutical excipients or cleaning product formulators select it to reduce the harsh footprint of their recipes. Rubber processing lines, especially in specialty elastomers, leverage its compatibility to avoid phase separation in complex mixtures. Each sector values it for a slightly different reason, but versatility keeps demand steady even when prices swing for related glycol-based products.
Researchers track ethylene glycol diacetate not just for its role as a solvent, but for improved sustainability in coatings and inks. New work points toward replacing toluene and xylene, both with greater health risks, with glycol-based esters in processes looking for greener portfolios. Laboratories at universities experiment with glycol diacetate’s use in microencapsulation and as a carrier for reactive monomers, aiming for safer alternatives in adhesives and sealants. Scientists looking to tweak the molecule’s performance play with chain length on related glycols, but always keep safety profiles in focus. In pilot projects, glycol diacetate often shows up where rules about worker exposure or plant emissions rule out traditional solvents, creating a market niche for small but crucial improvements.
Toxicological studies provide some peace of mind: the acute and chronic health risks for ethylene glycol diacetate fall below most aromatic hydrocarbons or lighter glycols, yet levels of concern remain. Animal studies point to mild irritation with repeated exposure, but little evidence supports higher carcinogenic or mutagenic risk at workplace concentrations. Inhalation exposures in laboratory mice and rats at doses much higher than human limits showed some liver and kidney impacts, though well within statistical ranges for organic solvents. Occupational safety officers monitor air quality around storage and mixing tanks, especially in workplaces where chronic low-level exposure accumulates. Industry and academia continue tracking metabolites, since breakdown products of related glycol esters sometimes show up in environmental samples if disposal corners get cut.
Solvent markets face a crossroads, where the balance between performance and regulatory demand tilts year on year. Demand for ethylene glycol diacetate shows reasonable strength as companies shift away from hydrocarbon-rich blends and look toward lower-toxicity options. Green chemistry labs test bio-based production from plant sugars, aiming to pitch sustainability alongside low odor and high solvency. Regulatory agencies in the EU and North America keep tightening volatile organic compound limits, so researchers hunt for new ways to extend glycol diacetate’s reach into coatings, ink jet printing, and specialty polymers. Processing advances and downstream applications, such as next-generation waterborne paints, will likely keep it in play, especially if new data strengthens the case for reduced health risk and improved environmental breakdown.
Ethylene glycol diacetate doesn’t roll off the tongue, and most folks don’t give it a thought, but it winds up in more places than expected. I first ran into it while reading the fine print on a paint can. Turns out, this stuff gives paint its staying power. Paint that dries too quickly leaves streaks and brush marks, and nobody wants to redo a whole wall. Ethylene glycol diacetate keeps the paint wet long enough to manage a smooth finish, especially important if you're covering a big living room.
It’s not just about making painting less frustrating. Think about markers and inks—many kinds, especially permanent markers, count on chemicals like this one. It stops the ink from clogging up the tip and drying out too fast. Most markers in my office drawer probably depend on ethylene glycol diacetate to stay fresh between uses.
Nail polish brings another example. Anyone who’s painted their nails knows the best brands don’t clump or get stringy mid-manicure. This chemical helps keep the polish workable, improving the odds of a smooth coat. It works behind the scenes in these cosmetics, doing the thankless job of making life a little easier.
Out in the industrial world, ethylene glycol diacetate really finds its niche. Manufacturers use it as a solvent in cleaning products, especially the kind meant to clean up greasy engine parts or stubborn adhesives. Factories don’t reach for plain water when grease and oil pile up. They grab cleaners packed with solvents that can break down whatever’s stuck. This chemical’s ability to dissolve both oil-based and water-based messes makes it a favorite for heavy-duty jobs.
Some coatings, adhesives, and resins call for a bit more subtlety than brute-force cleaning. Ethylene glycol diacetate steps in here, offering just enough solvent power without the harsh fumes or health risks that come with nastier chemicals. It's less smelly than many alternatives, which means working with it day in and day out is much less unpleasant for those on the factory floor.
Years ago, a friend in the local government started tracking workplace health issues related to solvents. We both learned many traditional solvents can cause headaches, dizziness, or worse if used indoors too often. Ethylene glycol diacetate holds an advantage thanks to its lower odor and relatively gentle effect on skin, compared to old-school options like toluene.
Safety comes up more now than ever. Factories and product-makers look for alternatives to solvents linked to health or environmental hazards. The unique properties of ethylene glycol diacetate let businesses stay productive and meet safety standards. Regulatory trends in the U.S. and Europe keep pushing for less toxic, less volatile chemicals. This one helps get the job done without setting off alarm bells.
Nothing’s perfect, and there’s always a trade-off. As more people worry about chemical runoff from manufacturing and household products, some turn their attention to solvents like these. The green chemistry movement constantly searches for new options made from renewable resources, aiming for cleaners and paints with fewer environmental drawbacks. Sustainable replacements that work just as well could eventually shift the balance away from classic solvents.
In the meantime, ethylene glycol diacetate supports a huge bunch of products and jobs. From personal experience, it makes home maintenance smoother, helps artists work longer, and lets factories put out better goods without risking worker safety. Chasing better, safer solutions—while respecting what already works—matters for anyone who cares about the stuff we use every day.
Bust open a drum of ethylene glycol diacetate—you’re greeted by a nearly invisible liquid with a mild, fruity scent. It’s not just for filling space. This solvent manages to slip into coatings, inks, and even flavors, all because of its mix of physical and chemical quirks. The clear appearance, even at room temperature, sends a message: stability and predictability headline its list of benefits.
In practice, I’ve noticed chemists and plant operators look for reliability in how a substance reacts to heat. Ethylene glycol diacetate boils at around 230°C—neither too eager to jump into vapor nor too stubborn to let go at reasonable industrial temperatures. This eases the way it’s handled in processes like distillation or solvent recovery. The freezing point drops out under -20°C. No need to worry about freezing on loading docks during winter—this property shaves headaches off logistics and storage.
Measuring density—1.127 g/cm³, if you want numbers—matters for anybody mixing or transporting chemicals. You wind up with a liquid that's heavier than water, which shows up in tank readings and pumping calculations. Viscosity isn’t just for chemists: pour a bit, and it moves smoothly, not syrupy, but not as runny as plain water. This makes filling, blending, and cleaning equipment less messy, improving turnaround time for maintenance crews.
Pick up a bottle, splash some in a beaker of water, and you’ll see why this property is more than trivia. Ethylene glycol diacetate dissolves easily in organic solvents. It takes more convincing to mix completely with water, but it manages enough solubility to matter. In the world of paint and coatings, this means better blending, smoother finishes, and fewer clumps—manufacturers like to keep surprises on the shelf, not in the can.
From my own work around labs and manufacturing sites, it’s clear that folks want a chemical that won’t throw curveballs. Ethylene glycol diacetate stays fairly inert in most cases. It holds up against the ravages of air and moisture; it doesn’t break down or react wildly unless pushed to extremes—like strong acids or bases, or heavy heat. With the right storage, containers stay in use for months, even years, without corrosion or dangerous leaks.
People don’t grab gloves and goggles for fun. Even a mild-smelling liquid like this one needs sensible handling: inhaling vapor or splashing it near the eyes triggers irritation. Studies list a relatively high flash point, so the risk of sudden fire is lower than for many solvents, yet good ventilation and spill controls stay important. Disposal gets attention too—nobody wants solvents clogging up waterways or damaging groundwater. That’s where responsible processing comes in, whether incineration or chemical treatment.
Manufacturers often look to ethylene glycol diacetate for thoughtful reasons. It makes coatings and inks dry just right, without leaving sticky residues. In flavors and fragrances, it hangs in the background without overpowering, letting other notes shine. Sometimes, labs turn to this solvent as a replacement for nastier or more expensive options. The challenge comes in recycling and disposal—technology for recovery keeps getting better, making this compound more appealing for folks looking to cut waste.
Storing and handling ethylene glycol diacetate isn’t the sort of chore you can just wing and hope it goes well. In my years working with different chemicals in various environments, I’ve seen how even the small choices—like grabbing the right gloves, double-checking labels, or choosing storage space thoughtfully—separate safe labs from disaster zones. The safety record on a shop floor or in a university setting never improves by accident. It builds from habit and clear respect for the material in front of you.
Take one look at ethylene glycol diacetate’s Safety Data Sheet, and you’ll spot the basics: dry, cool, and ventilated. No need to guess what happens if you slip up—a tight, hot, or damp spot causes pressure to rise, creating a mess that ruins both product and peace of mind. Putting this liquid in a steel drum isn’t enough. Use proper, airtight containers made of compatible materials and check for corrosion or leaks. I once saw someone use a cracked cap, thinking it’d last “just one more week.” Moisture crept in, and the resulting sludge told its story pretty quickly.
Temperature control remains a non-negotiable. Don’t store ethylene glycol diacetate near heat sources like radiators, direct sunlight, or where hot machinery runs. Fires start faster than people expect, especially with solvents. A cool room backed by industrial fans makes a solid investment. Many small operations forget ventilation, treating it as an afterthought. Once, I visited a warehouse reeking from unresolved spills. Poor air flow let vapors concentrate, and headaches spread across the crew. After they installed a new extraction fan, complaints faded and inspections grew easier.
Every use brings its own hazards. Splash goggles, nitrile gloves, and a decent apron all help curb risk. I always keep a pair of goggles and gloves as non-negotiable at the edge of the workbench. It’s tempting to think small spills can’t hurt, but seeing just one chemical burn changes that forever. Don’t trust bare skin, open-toed shoes, or improvised gear.
Pouring or transferring chemicals often causes the most trouble. Funnels, pumps, or even automated dispensers pay off by preventing splashes. Pour too fast, and you’ll end up cleaning a puddle instead of finishing a task. Even storing the right absorbent materials nearby shows that safety is part of the process, not a later fix.
People rarely expect accidents, and nobody likes emergency scenarios, but it’s surprising how fast training fades round the edges. I’ve watched new hires freeze in an alarm, unsure of the next step. Regular walk-throughs with fresh staff make a difference. Cleaning up a chemical spill or dealing with accidental exposure can go smoother with a few practiced drills instead of wild guessing under pressure.
Simple emergency measures save time and health: Know the nearest eyewash station, keep an updated first aid kit on hand, and post spill response sheets where folks can see them. One person who remembers to call for help or use the eyewash quick enough can make all the difference in a dangerous moment.
Ethylene glycol diacetate won’t cut corners for you, so don’t cut them on storage and handling. I’ve seen bad habits cost people their health and companies their reputation. Honest work, decent gear, and a little routine attention keep things running smooth—and let everyone head home safely.
You might not recognize the name ethylene glycol diacetate, but you rub elbows with chemicals like it all the time. Used in paints, cleaners, and coatings, this compound helps products dry more smoothly and stay workable longer. It sits on many ingredient lists in factories and workshops. I remember watching painters wipe down brushes, the sharp smell lingering in the air—often that’s a sign of solvents like this one.
Most folks growing up around hardware stores or working in auto shops can tell you about headaches, dizziness, and nose irritation after a day with solvents. Ethylene glycol diacetate isn’t some innocent bystander; it turns into ethylene glycol and acetic acid in your body. If too much builds up, problems like nausea, vomiting, and unsteady walking can creep in. In high doses, there’s a risk for kidney strain. Sure, folks use gloves and fans, but in the real world, personal protective gear sometimes gathers dust on the shelf. Without care, chronic low-level exposure adds up, just like years of sun can damage skin.
This chemical doesn’t simply disappear after use. Most factories do their best to handle leftovers, but spills and accidents happen. Runoff from cleaning tools can head down drains, eventually hitting rivers and streams. Ethylene glycol diacetate breaks down faster than some other solvents, yet its main breakdown product, ethylene glycol, isn’t so harmless to wildlife. Fish and frogs don’t get a say. A 2010 study from the EPA showed that moderate spills can poison water creatures and harm bugs that birds rely on for food. While it evaporates in open air, that doesn’t let our air off the hook. People living near busy plants sometimes notice the scents more sharply—a sign that chemical clouds hang especially heavy in still air.
We can’t just pull chemical helpers like these out of every product overnight without leaving industries in a bind. Coatings would dry too quickly, paint jobs would streak, and cleaners wouldn’t cut through grime as well. But it doesn’t mean shrugging our shoulders, either. In my experience working at a community hazardous waste collection event, people brought in buckets of half-used solvents with little clue about disposal. That’s a failure in communication from both companies and regulators.
Industry can shift to safer alternatives where possible. Water-based coatings and citrus-derived cleaners do the job in more places each year. For workers and folks at home, better labeling makes a difference. Instead of chemical jargon, warnings need plain language about ventilation, glove use, and safe disposal. If local governments offer drop-off days for hazardous chemicals, spreading the word beats the dangers of pouring something risky down the sink.
Regulations move the needle, but the real force comes from people who refuse to accept avoidable risk at work or at home. Few people read the small print, but most care about their families' health and about land and water close to home. My take: small shifts in knowledge ripple out. Better safety messages, smarter product choices, and strong community collection programs all blend to lower the risks. We’ve figured out how to face down lead paint and CFCs. With practical steps and less tolerance for business-as-usual, these less famous chemicals can follow.
Ethylene Glycol Diacetate (EGDA) often comes off the radar for folks outside the chemical world, but those working with solvents or specialty chemicals learn quickly how the wrong packaging can throw off an entire operation. At its core, this stuff is a clear, almost non-remarkable liquid—until you need it to stay pure and safe through shipping, storage, and months waiting on a shelf. I remember helping a small coatings manufacturer set up their warehouse; they’d stacked bulk drums under a leaky roof, and the headaches began three months later when some of the drums started to rust and leach. Sometimes, it’s all about packaging doing its job quietly in the background.
EGDA usually ships out in steel drums, often 200-liter capacity, or intermediate bulk containers (IBC totes) for the larger outfits. Steel makes sense because it keeps out most light and air, and the drums stay sturdy through plenty of rough handling. For smaller batches—a lab or shop-scale need—one-gallon or five-liter jugs with tight-sealing lids handle the job. Plastic works only if it resists chemical attack from esters in the long run, so folks skip the cheap stuff and stick with HDPE or fluorinated bottles. One manufacturer told me switching from unlined drums to epoxy-lined ones slashed their returns by half, especially for customers in humid climates.
A good seal is everything. I watched a barrel get dropped at a port in New Jersey, and the bung popped loose; within days, the contents absorbed water and picked up impurities. Companies with tight processes often use nitrogen blanketing before sealing drums—purging out oxygen and moisture. It might sound fussy, but one misstep in packaging and you’re dealing with off-spec solvent or safety recalls down the line.
Shelf life starts the clock from the day of filling. Most suppliers mark EGDA at about 12 to 24 months shelf life if it sits in unopened, original containers, at room temperature, and away from sunlight. That range isn’t just marketing—chemical breakdown or absorption of water through imperfect seals shaves time off the clock. Once someone cracks the seal, the timeline shrinks to a few months, especially in damp or hot environments.
You don’t need laboratory-grade controls, but I’ve seen that simple steps like stacking drums off the ground and keeping storage areas cool stretch the shelf life significantly. One plant manager in Georgia showed me his tracking log; batches stored past 18 months consistently failed their purity checks when drums were left out with fluctuating temps. Sticking to “first-in, first-out” saves both product and headaches.
Mistakes often happen in the handoff—from manufacturer to distributor to small business. The chemical itself won’t warn anyone if the packaging slips, but a quick look at the inner lining, the seals, and even the warehouse thermostat can make all the difference. Investing in better drum lining, keeping warehouse staff trained on the specifics of esters, and treating every delivery as perishable—these approaches can keep costs down. I’ve seen buyers ask for a certificate of analysis and ignore the drums’ manufacturing dates, only to pay the cost in degraded product later.
Ethylene Glycol Diacetate might seem simple, but attention to packaging and storage spells the difference between a reliable supply and wasted inventory. Clean containers, good seals, and a solid rotation plan outmatch last-ditch quality tests any day.
| Names | |
| Preferred IUPAC name | Ethane-1,2-diyl diacetate |
| Other names |
EGDA 1,2-Ethanediol diacetate Ethane-1,2-diol diacetate Glycol diacetate Diacetoxyethane |
| Pronunciation | /ˌɛθ.ɪˌliːn ɡlaɪˈkɒl daɪˈæs.ɪ.teɪt/ |
| Identifiers | |
| CAS Number | 111-55-7 |
| Beilstein Reference | 1840627 |
| ChEBI | CHEBI:40709 |
| ChEMBL | CHEMBL1689629 |
| ChemSpider | 13408 |
| DrugBank | DB13917 |
| ECHA InfoCard | 100.008.190 |
| EC Number | 203-892-1 |
| Gmelin Reference | 150814 |
| KEGG | C19648 |
| MeSH | D004991 |
| PubChem CID | 7518 |
| RTECS number | KW2975000 |
| UNII | 50A86606F3 |
| UN number | “UN3272” |
| CompTox Dashboard (EPA) | DTXSID9086523 |
| Properties | |
| Chemical formula | C6H10O4 |
| Molar mass | 146.141 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild, pleasant, ester-like |
| Density | 1.095 g/cm3 |
| Solubility in water | 10 g/L (20 °C) |
| log P | 0.18 |
| Vapor pressure | 0.013 mmHg (20 °C) |
| Acidity (pKa) | 14.4 |
| Basicity (pKb) | pKb: 15.3 |
| Magnetic susceptibility (χ) | -84.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.415 |
| Viscosity | 1.69 cP (25°C) |
| Dipole moment | 4.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 356.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1104.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2046.7 kJ/mol |
| Pharmacology | |
| ATC code | V07AY24 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| Flash point | '122 °C' |
| Autoignition temperature | 224 °C |
| Explosive limits | Explosive limits: 1.7–10.3% |
| Lethal dose or concentration | LD50 (oral, rat): 5,600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 8,500 mg/kg |
| NIOSH | KWG35300 |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol Ethylene glycol diethyl ether Ethylene glycol monoacetate Diethylene glycol diacetate Propylene glycol diacetate |