Ethylene glycol di methyl ether, often showing up as monoglyme or dimethoxyethane in lab catalogs, started carving out its place in the chemical world during the mid-20th century. Early synthetic chemists caught onto its knack for dissolving a wide range of polar and nonpolar compounds, making it a staple for organometallic reactions. As more researchers tweaked lithium batteries and dived into polymer chemistry, lab benches began piling up bottles of this ether alongside other glycol derivatives. Its footprint in technical literature keeps growing, especially where researchers demand solvents that stand up to tough conditions yet keep reactions under control.
Monoglyme’s clear, nearly odorless appearance looks unremarkable, but anyone who has worked in a lab understands the difference between a handy solvent and one that unlocks new types of reactions. This compound waves off water fairly easily and handles heat better than many counterparts. Industrial players rely on it not just for classic extractions, but as a reaction medium for complex hydroboration, polymerization, and reduction work where keeping water away can make or break the end result. Its popularity extends from small-scale synthesis to serious commercial production runs.
Ethylene glycol di methyl ether rolls in as a low-viscosity liquid with a boiling point hovering near 85 °C and a freezing point close to -58 °C. The compound weighs in at about 90 g/mol and boasts a density around 0.86 g/cm³. It mixes easily with most organic solvents and water—two qualities that smoothen many tricky separation steps. In my own work, its sharp solvating abilities have untangled plenty of stuck reactions, and it rarely gets fussy about impurities, unlike more sensitive ethers. Flash point, at about 5 °C, does mean it catches fire quite easily, so care never takes a back seat in any environment.
Chemical suppliers print lots of specifications on each drum: purity (often above 99%), water content, and residue after evaporation. Labels emphasize industrial hygiene standards with hazard pictograms and signal words such as ‘Danger’ due to its flammability and toxicity. A quick scan of a safety data sheet shows information about proper storage, recommended handling temperatures, and shelf life. Having spent time in shared research labs, clear labeling and color-coded drums help prevent disastrous mix-ups—a necessary safeguard when even a tiny splash or vapor can set off major incidents.
Production often involves condensation of ethylene glycol with methanol under acid catalysis, usually leveraging efficient dehydration conditions to push the reaction forward. Some plants use distillation columns running under reduced pressure to minimize side reactions and achieve higher purity. In small-scale work, distilling over sodium keeps water and peroxides at bay. Labs that lack fine-tuned controls often end up with higher impurity content, which can spoil sensitive downstream chemistry. Choosing sources with solid QC backgrounds saves headaches in later applications.
Holding two methoxy groups, this ether reacts with strong acids, breaks down under vigorous oxidation, and acts as a ligand for stabilizing alkali metals. Lithium battery research leaned heavily on this ability, as solvated electrons in ethereal environments become more workable. It supports Grignard and other organolithium reactions, enabling formation of carbon–carbon bonds critical to new drug candidates or specialty polymers. In my own hands, swapping monoglyme for another ether flipped yields by double digits, underlining how much it influences outcomes, especially when dealing with moisture-sensitive or highly reactive intermediates.
Ethylene glycol di methyl ether pops up on inventories as monoglyme, 1,2-dimethoxyethane (DME), glyme, or even EGDME. Distributors like Sigma-Aldrich, Alfa Aesar, and Tokyo Chemical Industry list it under all those titles, depending on purity or targeted use. Purchasing under the right name means suppliers line up specifications with the experiment’s needs, stopping confusion that could derail time-sensitive runs or scale-up operations.
Handling rules never get ignored. Chemical splash goggles, flame-retardant lab coats, and good ventilation come into play due to its high volatility and tendency to form explosive peroxides. Reports link monoglyme exposure to nervous system effects and blood toxicity. NIOSH and OSHA lay out tight exposure limits: proper storage in sealed containers, away from heat, ignition sources, and oxidizers, is non-negotiable. Workers track odor thresholds and regularly test for air concentrations in closed spaces. Even after years of handling it, I won’t turn my back, since a careless move can tip conditions from safe to hazardous in seconds.
In battery research, monoglyme contributes by stabilizing lithium salts and flattening out cycling curves in cell development. Pharmaceutical chemists rely on it for its mild, accommodating solvency profile, making certain multi-step syntheses of active drugs more manageable. Paint and coatings industries use it as a flow agent or moisture scavenger. In electronics, workers use it to wash and prep surfaces needing strict standards of cleanliness. My old colleagues in start-up labs pointed out that using monoglyme over less effective counterparts trimmed purification cycles and cut raw materials usage—a big deal for tight budgets.
Researchers keep searching for alternative solvents to reduce worker exposure and environmental impact. Still, monoglyme sticks around since it delivers reliability across the board. Every year brings out fresh papers outlining its use in cutting-edge catalysis and green technology. Innovation in battery electrolytes continues feeding demand for well-characterized, stable ethers. Projects exploring flow-battery prototypes and next-gen organic solar panels often feature monoglyme as a core process solvent.
Long-term studies stack up health risks associated with repeated exposure. Data point at hematological toxicity, with a worrying effect on red blood cell production. Animal studies showed reproductive toxicity and organ damage after chronic high-dose exposure. These findings pushed agencies to flag it on regulatory lists, driving tighter controls on workplace exposure. Modern facilities invest in scrubbers, automated pipetting, and air monitoring to minimize risk—a lesson learned by watching too many cases of workplace exposure pile up. Substitution remains an area of active review, despite few direct drop-in alternatives with the same performance.
Calls for safer, greener solvents weigh against monoglyme’s proven track record. Some innovators chase biobased ethers with similar chemistry, but scaling up and matching performance still present challenges. Regulatory pressures spur development of closed systems and smart monitoring tools. With continued investment in batteries and specialty pharma, strong demand persists, provided manufacturers and users keep safety and environmental impact front and center. Newer regulatory frameworks will likely force a fresh round of process changes or new safety hardware, but as long as product synthesis and tech innovation rely on robust, reliable solvents, ethylene glycol di methyl ether keeps its seat at the lab bench, at least for now.
In chemistry labs, Ethylene Glycol Di Methyl Ether goes by a shorter name—diglyme. Chemists, especially those in research or manufacturing specialties, become familiar with it well before graduation. You won’t find diglyme in your average hardware store aisle. In industries working with electronics, pharmaceuticals, or even specialty plastics, diglyme tends to be a steady presence, popping up wherever precision and reactivity matter.
Ask anyone who’s had their hands in chemical synthesis about reliable solvents, and diglyme usually makes the shortlist. Unlike more common solvents like ethanol or acetone, diglyme provides a rare combination of low volatility and strong solvency. In the lab, it often supports the preparation of Grignard reagents—compounds critical for putting together complex molecules. Every year, these help researchers build everything from custom polymers to life-saving drugs.
The unique structure of diglyme allows it to dissolve both polar and non-polar substances. This means it can break up salt residues while still carrying out delicate organic reactions. Equipment clean-up, reactions that need just the right temperature, even high-purity processing—diglyme’s chemistry fits the bill where other options might fall short or create hazards.
Electronics manufacturers rely on diglyme in their electrolyte solutions for lithium batteries. Its stability holds up under the high demands of battery cycling, helping power up smartphones, laptops, and electric cars. In semiconductor fabrication, diglyme gets used as a cleaning and etching agent. These facilities depend on solvents that don’t leave behind residues and won’t react unpredictably with sensitive materials.
On the pharmaceutical side, diglyme shows up in the crafting of antibiotics, cardiovascular drugs, and specialty intermediates. Some drugs need extremely clean reaction environments—diglyme offers that clean slate. Chemical engineers don’t like surprises, and diglyme’s predictability gives them fewer headaches, keeping batches both safe and consistent.
Handling diglyme does require extra caution. The chemical’s ability to dissolve a wide range of substances is great for industrial purposes but not so friendly to human tissue or aquatic life. Without proper ventilation or protective equipment, exposure can irritate eyes, skin, and even central nervous systems. Over the past decade, European regulators have called out diglyme for its reproductive toxicity, and limits have tightened in some jurisdictions.
Waste disposal adds another layer. Labs and factories producing large quantities of chemical waste face the challenge of keeping diglyme out of water supplies and soil. In places with less oversight, improper disposal risks big environmental damage.
People invested in safer labs and plants look for greener options, but replacing diglyme isn’t easy. Many alternatives don’t stack up for performance or cost. Researchers keep poking at the problem, redesigning reactions to use less, recycle more, or swap drop-in substitutes for diglyme. Battery makers test new solvents, hoping not to sacrifice power or reliability for safety. Legislators and watchdog groups push for clearer guidance around handling and cleanup.
End-users—the ones designing new circuits, developing drugs, or assembling the gadgets on our desks—play a real part too. Keeping pressure on suppliers for transparency and greener chemistry often helps shift the balance toward safer workplaces and healthier neighborhoods. In the end, the push for practical, safe chemicals touches everyone connected to manufacturing and high-tech research.
Ethylene Glycol Di Methyl Ether shows up in labs and industries where folks sometimes don’t give its risks much thought. Years back, I saw a co-worker pour some out without gloves, only to end up with red, irritated skin by lunch. It hardly takes a spill to see problems—this chemical absorbs fast, and skin contact doesn’t just sting, it brings on tougher health issues over time. Reading stories or watching someone learn the hard way sets the reminder: never skip those gloves or goggles, no matter how much of a rush the day brings.
No one wins by skipping personal protection. Decent nitrile gloves, a lab coat, and a pair of safety goggles aren’t overkill. Splashing some of this stuff in your eye could cause lasting damage, and once inhaled, vapors can leave you dizzy or foggy-headed. Inhaling even a little on a regular workday drags down focus and, in harsh cases, raises the risk of kidney or liver trouble over time. I’ve had to nudge plenty of colleagues, “Slap those gloves on,” before starting a project—they’re a bit annoyed for a second, but thankful later.
No one wants to work in cramped places where vapors hang in the air. Ethylene Glycol Di Methyl Ether gives off fumes with room temperature use, so staying away from open beakers on benches helps. Throwing open a window isn’t enough—good lab fans or fume hoods make a world of difference. I once tried short-cutting and poured just a splash in a closed-off corner; a headache kicked in that didn’t leave for the whole afternoon. Engineers and lab managers need to set up airflow systems to keep everyone on the safe side. That might mean springing for new exhaust systems, but that cost pays off.
Storing this solvent calls for clear labels and solid containers, tightly shut and far away from open flames or sparks. Ethylene Glycol Di Methyl Ether burns easily and reacts badly to air, sometimes forming nasty peroxides if left old or uncapped. A friend found herself wrestling with a jammed bottle whose top had crystallized—luckily she stopped, but it could have ended much worse if she’d forced it open. Sticking to proper inventory means checking expiration dates and handling containers that seem off with caution. OSHA encourages folks to limit how much they keep around to what’s actually needed, and that approach avoids bigger pile-ups and headaches.
Getting the basics in place will never replace the kind of culture where everyone looks out for each other. Regular training and real conversations turn rules on a wall into habits. New students and seasoned pros alike should talk over safe handling steps and share stories about what went sideways. Quick spill clean-up kits, well-marked emergency showers, and clear exit plans stand out in any sensible workspace. It’s easy to forget the risks in daily routines, so reminders and simple checklists go further than you'd expect.
Better habits kick in when folks own their part of the safety process. From supervisors pointing out proper gear, to teammates watching each other’s back, handling Ethylene Glycol Di Methyl Ether safely starts with showing you care. Take the time to check that nothing’s been overlooked: gloves in the right place, fume hoods switched on, bottles checked for integrity before use. That little extra effort protects everyone, and every lab accident dodged proves the value of not cutting corners.
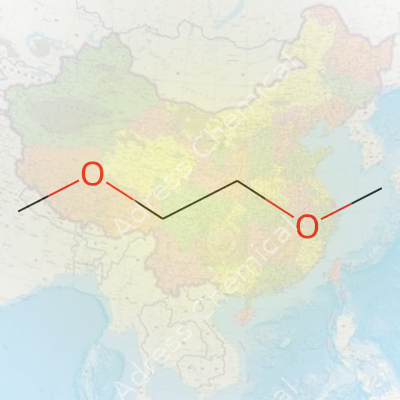
Ethylene Glycol Di Methyl Ether, known by the mouthful name 1,2-Dimethoxyethane (or DME and Glyme in the chemistry world), carries the formula C4H10O2. On paper, this reads as four carbons, ten hydrogens, and two oxygens. The structure is simple: two methoxy (-OCH3) groups bookending an ethylene (–CH2–CH2–) bridge. You end up with CH3O–CH2CH2–OCH3. Imagine a two-carbon chain holding hands with two oxygen atoms, each linked to a methyl group—no rings, no loops, just a straight link-up.
Years ago, during some hands-on work in a university lab, I kept bumping into DME as a solvent that made tough reactions possible—especially those involving lithium. The magic comes from the two oxygen atoms. They grip cations like lithium or sodium, almost like a jacket zipped in tight. Because of these guests, reactions that sputter in basic solvents get a second wind with DME. I remember my mentor uncapping a bottle of DME and showing how quickly it mixed with water, yet never got sticky like syrupy ethylene glycol.
Anyone spending time around glycol ethers knows the safety spiel by heart. DME brings flammability to the table, so care means proper lids, a well-ventilated fume hood, and no open flames. Even chemistry students hear the stories about ether fires. Beyond the fire risk, frequent exposure should stay on everyone’s radar because glycol ethers can wander in the bloodstream and spark health troubles. DME loves to soak through skin, and headaches or dizziness remind you to keep the gloves on. Safety Data Sheets stick to my workspace wall, not as warnings, but as reminders born from stories passed between chemists who learned the hard way.
Take electronics and batteries—fields obsessed with keeping water away. DME steps in because it dissolves lithium salts and other electrolytes. The solvent opens doors for lithium batteries powering phones and electric vehicles, or for the more niche world of high-performance capacitors. It’s not only scientists who count on DME’s reliability. Industries that process synthetic fibers, resins, and dyes have also woven it into production lines.
Every time I see cutting-edge battery research, DME is rarely far from the scene. I’ve watched research teams battle humidity in makeshift labs, reaching for DME as their ‘get out of jail’ card because water gets in the way, and DME’s lack of hydrogen bonding simplifies cleanup and maximizes control.
Left unchecked, solvents like DME can follow factory effluent into water systems. Even in low doses, ethers mess with aquatic life and have a way of sticking around. Cleaner disposal and minimizing spills aren’t just regulatory box-checks—they matter in the long run. Labs and industries have pushed adoption of recovery systems, reusing DME by distillation instead of dumping it. This approach shields ecosystems but also slashes costs and chemical waste.
On the design side, chemists are hunting for safer alternatives offering the same solvating power, but with fewer environmental bruises. Switching to water-based or less persistent solvents isn’t easy, especially with sensitive reactions. It demands innovation not just in the flask, but up the chain—from manufacturers to R&D. These efforts don’t always make headlines, but they quietly shift habits and keep both science and safety moving forward.
Ethylene Glycol Di Methyl Ether isn’t a household name. In some industries, folks know it as Monoglyme. Solvent users count on it to dissolve all sorts of things and to keep certain reactions going. Still, that clean and clear liquid brings real hazards into a workspace. Breathing its vapor, letting it splash on skin, or accidentally lighting it up could cause trouble. So, talking about its safe storage strikes me as more than a box-ticking exercise—it's about looking out for health and keeping workspaces off a headline for the wrong reasons.
Storage gets complicated because ethylene glycol di methyl ether evaporates pretty fast. The fumes don’t just disappear—they can pool and hang in the air, raising the risk of headaches, dizziness, or worse. The fire risk shoots up when vapor meets an ignition source. I’ve seen cases where stored solvents turned small spills into big emergencies, all because lids weren’t tight or storage areas sat near heat.
Start by finding a spot away from sunlight, heaters, and sparks. This isn’t picky—just practical. If the storage room feels too warm for comfort, conditions are likely slipping into danger. Metal safety cabinets are worth their cost; they keep fumes inside, even if someone accidentally tips the bottle. My go-to move: label every container. If folks can’t quickly tell what’s inside, accidents wait in the wings.
Speaking from experience, ventilation never feels important until something goes wrong. People sometimes keep bottles in closets, thinking it keeps things safe and out of sight. Yet, without a fan or vent, fumes hang around. Instead, choose a spot with steady air movement. If you can smell chemical odor, that’s a sign to rethink how air flows in that area.
Some solvents chew through labels, lids, or even some plastics. Ethylene glycol di methyl ether belongs in tough, chemical-resistant bottles. Glass with tight lids or certain plastics work better than old, reused bottles with mystery histories. Never store it in an old soda or water bottle. Someone might mistake it for a drink, which brings a whole new set of problems nobody wants.
Putting chemicals on high shelves or out-of-reach areas doesn’t improve safety. If a person tries to pull a bottle down while juggling other items, spills seem nearly guaranteed. Keep containers at a reachable height, away from the edge, and with enough space between them to avoid collisions. Spill trays underneath bottles make cleanup easier if something leaks.
Storing dangerous chemicals always relies on people, not just shelves and bottles. Teach anyone working nearby what to do if a spill happens or if someone feels sick from fumes. Let everyone know where to find gloves, goggles, and proper cleanup tools. Working without a spill kit or thinking “someone else will take care of it” leads to chaos.
In many workshops and labs, old habits slip in—a bit more storage here, a shortcut there. Tight schedules don’t excuse risky decisions. Refresh your space regularly. Sweep, declutter, and update containers. Pay attention to expiration dates. If a bottle looks odd, don’t guess—replace it. Good storage isn’t glamorous, but everyone benefits when people take it seriously.
Ethylene glycol dimethyl ether, also known as monoglyme, has a spotty reputation in labs and industry for a reason. It serves as a solvent in batteries, electronics, and pharmaceutical work. Plenty of people, myself included, have seen it show up in experiments and technical manuals. The catch comes down to what it does if it escapes that neat bottle or gets into the wrong place.
Nobody really wants to handle chemicals that can sneak into your system through the skin, yet monoglyme fits the bill. Even wearing gloves doesn't completely erase the risk if they're the wrong type. Years ago, a chemist friend of mine brushed up against a tiny spill and brushed it off—literally—and regretted it later. Skin rash, headache, nausea appeared soon after. Monoglyme gets absorbed and causes trouble, especially for the nervous system and blood. Extended exposure brings more serious risks, from dizziness all the way to possible liver and kidney issues.
Lab safety data sheets point to reproductive concerns with this compound. Studies on animals—rats and mice—show birth defects and fertility effects. It’s not just a theoretical risk. Countries like the UK have put strict limits on workplace air concentrations. OSHA in the United States flags it for monitoring in workplaces, since chronic exposure over time can build up damage.
The environmental story isn’t much brighter. Monoglyme’s high solubility in water lets it slip into groundwater and rivers quickly. Once it’s loose, the breakdown process crawls. Aquatic life—think small fish, plankton, amphibians—faces an uphill battle if concentrations rise. Even low levels can disrupt growth and development.
A few years back, there was a minor panic near a battery manufacturing site after local water showed trace amounts. Fish populations took a hit. Local farmers had contaminated irrigation lines that needed heavy flushing and remediation. Cleanup adds cost and worry for companies and communities alike.
Substituting monoglyme with less hazardous solvents sits high on the wish list. Labs have switched to alternatives like dimethyl sulfoxide or propylene carbonate for certain uses, cutting personal risk to chemists and reducing environmental leaks. That kind of change takes time and investment, though, and doesn't work for every process.
Folks working with monoglyme need real protection—proper gloves, fume hoods, good ventilation. It’s not enough to stash the stuff in a locked cabinet. Regular air monitoring catches leaks early, and spill kits save headaches down the line. Companies benefit by training staff the right way, not just handing out manuals.
Some progress comes from regulations, but enforcement makes the actual difference. Tough rules only matter if inspections and penalties follow up. Community reporting hotlines give neighbors some power and make bad actors think twice.
Nobody wants tighter rules just for the sake of paperwork, but everyone likes clean water and healthy workers. Companies keep a closer eye on their emissions now, knowing bad press and lawsuits come quickly. Labs have started sharing safer alternatives, and younger chemists often push for greener solutions from day one. Every step away from dangerous solvents like monoglyme pays off in fewer sick days, cleaner neighborhoods, and peace of mind for people who just want to do their jobs safely.
| Names | |
| Preferred IUPAC name | 1,2-Dimethoxyethane |
| Other names |
Dimethoxyethane Glyme Monoglyme |
| Pronunciation | /ˌɛθ.ɪˈliːn ˈɡlaɪ.kɒl daɪ ˈmɛθ.ɪl ˈiː.θər/ |
| Identifiers | |
| CAS Number | 110-71-4 |
| 3D model (JSmol) | `3D model (JSmol)` string for Ethylene Glycol Di Methyl Ether: ``` COCCO ``` |
| Beilstein Reference | 1361113 |
| ChEBI | CHEBI:40451 |
| ChEMBL | CHEMBL133933 |
| ChemSpider | 10736 |
| DrugBank | DB14019 |
| ECHA InfoCard | 13b2ac7a-61d2-4a76-8b72-7e67e92ce409 |
| EC Number | 203-794-9 |
| Gmelin Reference | 6467 |
| KEGG | C01133 |
| MeSH | D004992 |
| PubChem CID | 8097 |
| RTECS number | KL5775000 |
| UNII | TCX96411YN |
| UN number | UN2372 |
| CompTox Dashboard (EPA) | DTXSID1020342 |
| Properties | |
| Chemical formula | C4H10O2 |
| Molar mass | 90.12 g/mol |
| Appearance | Colorless liquid |
| Odor | ether-like |
| Density | 0.867 g/cm3 |
| Solubility in water | Miscible |
| log P | -0.36 |
| Vapor pressure | 3.7 kPa (at 20 °C) |
| Acidity (pKa) | 37.0 |
| Basicity (pKb) | pKb: 0.21 |
| Magnetic susceptibility (χ) | χ = -44.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.378-1.381 |
| Viscosity | 0.474 mPa·s (at 25°C) |
| Dipole moment | 1.15 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 225.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -368.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3042 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | D08AX99 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302, H319, H332, H360FD |
| Precautionary statements | P210, P261, P280, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | “-1 °C (30.2 °F) (closed cup)” |
| Autoignition temperature | 160 °C (320 °F) |
| Explosive limits | 2.4% - 23.5% |
| Lethal dose or concentration | LD50 (oral, rat): 7.2 g/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat 7,238 mg/kg |
| NIOSH | NIOSH: KK8125000 |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Dimethoxymethane Diethylene glycol dimethyl ether Diglyme Diethyl ether Dimethyl ether |