Before anyone tossed the term Dipropylene Glycol Propyl Ether into a conversation, people relied on a grab bag of solvents, many of which were either too strong, smelly, or left a mess behind. The real spark for development of propyl ethers began in the late 20th century, right as regulatory pressure started turning up the heat on older, harsher solvents. Chemical makers wanted something with a lighter environmental footprint, improved worker comfort, and solid performance in everything from cleaning agents to paints. Dipropylene Glycol Propyl Ether emerged out of this challenge—offering a middle ground between aggressive solvents and water. The stuff started popping up in technical papers and trade journals soon after, as researchers and companies looked for something safer, something less likely to catch fire, and something with just the right volatility.
What rolls off the tongue as “DPGPE” quietly handles day-to-day tasks in coatings, inks, floor polishes, industrial cleaners, and even some personal care formulas. Plenty of laboratories keep it stocked, not because it’s flashy, but because it just works. Its balance between hydrophilic and hydrophobic properties gives it the ability to dissolve grease, resins, and other sticky foes where a straightforward alcohol or water won’t do the trick. Compared to long-forgotten predecessors, DPGPE doesn’t hit the nose with harsh odors or leave behind stubborn residues. End users aren’t always aware that many water-borne formulations lean on this ingredient just to stay mixed, stable, and easy to spread.
A clear, colorless liquid at room temperature, Dipropylene Glycol Propyl Ether carries the formula C9H20O3 and a molecular weight around 176.25 g/mol. Its boiling point lands north of 230°C, ticking off the requirements for a high-performance, low-volatility solvent. Low viscosity, paired with a flash point above 100°C, brings some peace of mind when the workplace heats up. DPGPE blends readily with water and many organics, crossing boundaries that water or stronger solvents alone can’t manage. Its moderate evaporation rate makes it a steady presence in any film-forming or cleaning system. The low odor profile goes a long way on factory floors, especially compared with traditional glycol ethers that could overwhelm workers.
Industrial buyers often look for purity above 98%, backed by gas chromatography or similar techniques for quality assurance. Labels across suppliers highlight its CAS number (29911-27-1), flash point, boiling point, density reports, and water content. Safety data sheets include solvent compatibility, recommended storage temperatures, and ways to avoid unwanted reactions—sticking mainly to dry, well-ventilated conditions and keeping sources of ignition at bay. Certifications for Reach, TSCA, and other compliance marks usually keep the paperwork rolling, particularly for companies exporting finished products that count on this glycol ether.
Manufacturers typically prepare Dipropylene Glycol Propyl Ether by reacting propylene oxide with propanol in the presence of a catalyst, then distilling and cleaning up the mixture. This process spits out a blend of isomers; those with the right boiling points get separated in a fractionating column. Control over temperature, pressure, and catalyst types affects yield and purity, which matters for downstream customers using DPGPE in sensitive formulations. High-purity stems from well-maintained equipment and vigilant monitoring during the reaction and distillation stages.
DPGPE itself sits at the crossroads of chemical functionality: the ether and alcohol groups make it reactive enough for esterification or further alkoxylation, but stable enough to survive harsh conditions in paint booths or large-scale cleaners. Labs can tweak the molecule by extending the propylene glycol chain or swapping the propyl group for other alkyls to tune solubility, volatility, or compatibility. Hydrolysis can split it back into propylene glycol and propanol, especially under strong acid or base conditions. The molecule doesn’t go around oxidizing easily, but exposure to air and heat for long periods can bring out byproducts, which typically stay below regulatory thresholds if handled properly.
Nobody wants to stumble over “Dipropylene Glycol Propyl Ether” every time something needs to be ordered or filed. In practice, you’ll hear trade names like Dowanol DPnP, Arcosolv DPNP, or Propasol PDP floating around in industrial circles. Chemists may jot down DPnP, DPGPE, or the longwinded 1-(2-propoxy-1-methylethoxy)-2-propanol on labels and technical sheets, especially if the compound could be confused with related glycol ethers. These synonyms help avoid costly mix-ups, especially in global trade where a single letter can mean a different chemical entirely.
Handling DPGPE in a shop or plant means keeping an eye on both worker health and process safety. Contact with the liquid can cause mild eye or skin irritation for some people, so gloves and goggles show up in standard safety kits. Good ventilation keeps any vapor levels below occupational limits, which, according to ACGIH and OSHA, stay relatively high compared to more volatile or aggressive solvents. DPGPE doesn’t catch fire as easily as lower alcohols, but any chemical storage area needs proper grounding, spill management, and leak detection—particularly in mixed-use settings where oxidizers or acids get stored nearby. Fire protection procedures call for foam, dry chemicals, or CO2 rather than plain water.
Cleaning products often carry the torch for DPGPE thanks to the ether’s ability to bridge water and grease. Formulators looking for better streak-free glass cleaners or low-odor floor polishes find this glycol ether pulls its weight. Paint and ink industries rely on its slow evaporation and strong solvency for colorants and resins. In coatings, DPGPE plays a role in lowering viscosity without thinning the final film or messing up gloss. Some metalworking fluids and specialty lubricants run more smoothly with a touch of DPGPE, letting workers cut metals or stamp sheet goods with less mess and downtime. Even cosmetics manufacturers reach for it to help fragrances mix and lotions lay down more smoothly, with personal care safety standards guiding every step.
Academic papers and industry-led studies keep finding new angles with DPGPE—better ways to blend it with bio-based ingredients, ways to improve drying times in water-borne paints, or opportunities to boost extraction power in tough separation processes. Researchers constantly test its compatibility with pigments, surfactants, and less conventional additives, searching for new uses in textiles, adhesives, or electronic cleaning. In labs where green chemistry takes center stage, some teams have looked at ways to make DPGPE using renewable feedstocks or combining it with biodegradable polymers, aiming for performance without as many environmental tradeoffs.
Big agencies around the globe keep a watchful eye on glycol ethers, and DPGPE’s relatively low acute toxicity keeps it in good standing for most industrial and household uses. Animal testing and cell studies point to low-level irritation as the most likely fallout from inappropriate handling, with little evidence for carcinogenicity or reproductive toxicity in standard usage scenarios. Chronic exposure studies continue as chemists look for any hint of long-term risk, especially as products containing DPGPE reach broader markets worldwide. Product stewardship groups work to update guidelines and review data, driving both transparency in labeling and new engineering controls where workers handle large volumes.
Demand for Dipropylene Glycol Propyl Ether seems set to keep rising, as more industries kick old-school solvents to the curb and lean into options with fewer regulatory headaches and better worker acceptance. The growth of waterborne coatings, adhesives, and cleansers depends on solvents like this that can manage both organic and aqueous loads. Innovation will keep pushing at the boundaries—bio-based propylene oxide, more efficient purification steps, smarter ways to capture and recycle fugitive emissions—all stand to make DPGPE a more sustainable choice. As regulatory requirements tighten and consumer awareness of chemical safety keeps climbing, companies that get ahead with cleaner formulations and transparent handling will keep DPGPE in the spotlight for years to come.
Dipropylene glycol propyl ether isn’t a phrase most people toss around at dinner, yet its fingerprints are all over our homes, offices, and even factories. This chemical plays a big supporting role in making many modern products work just right. If you’ve used a spray cleaner that didn’t streak, or stripped old paint without fumigating the whole house, you’ve probably crossed paths with this stuff.
What matters most for folks making paints, cleaners, inks, and coatings is performance. They need something that dissolves greasy grime, helps blend stubborn ingredients, and dries without leaving behind marks. This glycol ether handles those jobs well. It blends smoothly with water and a wide range of organic solvents. This means it can tackle oil-based messes and water-soluble gunk in one swoop. Take glass cleaners: customers flip if they see haze left after wiping, or if cleaners dry too slowly and drip down surfaces. Makers add dipropylene glycol propyl ether because it helps dissolve the grime, suspends particles so they can wipe clean, and then dries at a pace just quick enough to avoid streaks.
In the world of paints and coatings, nobody wants to repaint because of bubbles or brush marks. This chemical stretches out the drying time just a bit, giving paint or varnish more time to flatten and settle. For industries making high-performance inks for printers or markers, dipropylene glycol propyl ether keeps the ink from clogging up inside pens, helps lay down smooth color, and doesn’t leave nasty fumes that workers don’t want to breathe.
Digging a bit deeper, any chemical used this widely needs a clear safety profile. Contractors cleaning old walls, or families mopping floors, don’t want headaches or rashes from hidden dangers. Regulators in the US and Europe have looked at this compound closely. At typical levels in consumer and professional products, it’s considered safe with proper ventilation and skin protection. Even so, long-term, high-level exposure could carry risks, so any manufacturer worth their salt trains workers and shares clear safety sheets.
Consumers push for greener, safer products every year. More companies have switched to ingredients like dipropylene glycol propyl ether because it works well and lets them lower volatile organic compounds (VOCs) in their formulas. Fewer VOCs mean less smog in busy cities and healthier indoor air. Scientists keep tweaking these formulas, searching for options that use renewable resources or even safer alternatives, but for now, this glycol ether handles a lot of the jobs that keep modern cleaning and painting safe and effective.
If you flip through ingredient lists on cleaning sprays or paint cans, you might find this glycol ether under a few different trade names. Its smooth compatibility with water and oils helps it fly under the radar while quietly making products work better. Choosing products with lower VOCs, solid safety testing, and transparent labeling—these steps matter just as much as reading the fine print on the chemicals inside.
Most of us only notice chemicals like Dipropylene Glycol Propyl Ether (DPPnP) through household cleaners, paints, or industrial products. Yet, the discussion about safety always follows right behind, and for good reason. DPPnP belongs to a family of glycol ethers that help dissolve stains, grease, and dirt, making cleaning products much better at their job. Because it plays such a big role in that daily grind, knowing what we’re inviting into our homes and workplaces matters.
I once ran a small commercial cleaning business. We worked long hours with industrial chemicals, including various glycol ethers. You remember the products you use because they can leave a mark. Some would make your hands red or trigger a sneezing fit if you forgot to wear a mask. So when the term “safe” comes up, I think of the people on the frontlines—cleaners, painters, plant workers—who touch and breathe in these products every day.
DPPnP earns praise because it has lower odor and less irritation compared to other solvents. Studies cited by the US Environmental Protection Agency and European Chemicals Agency show that most exposures in consumer products fall well below thresholds tied to irritation or chronic effects. In lab tests, skin contact and inhalation only turn risky at much higher doses than you would typically hit, even with frequent cleaning. That matches my experience—keeping gloves handy is smart, and using a fan helps a lot, but most people don’t react unless they’re dealing with large spills or ignoring simple safety steps.
Researchers have looked at DPPnP for acute toxicity, chronic effects, and potential to disrupt the environment. Oral toxicity in rats sits at relatively high thresholds, meaning accidental exposure is unlikely to cause harm in small amounts. Direct skin or eye contact with the pure chemical can cause redness and discomfort, but diluted solutions in household products are much milder. Inhalation studies point to low risk under normal conditions.
Regulators do not assign DPPnP to high-risk categories. The U.S. EPA lists it as a solvent with “low to moderate” concern, mainly tagging long-term, high-level exposure as a point for extra caution. Europe’s ECHA shares a similar outlook. Both stress using gloves, goggles, and working in well-ventilated areas—recommendations that protect against almost all solvents. The Centers for Disease Control and Prevention notes that proper ventilation and protective gear will handle most of the safety concerns, based on existing research.
Safety comes down to habits as much as chemical properties. My teams learned early to never skip gloves or leave lids off bottles. Training was not just a formality; it was a way to make sure no one developed rashes, coughs, or headaches. Anyone using DPPnP—at home or on the job—should check the label for dilution, follow the product’s directions, and bring in fresh air. Switching to less hazardous products when possible also helps, especially in places with kids, pets, or people with asthma.
Open conversations about what’s in our products raise the bar for everyone. The facts point to DPPnP being a safe option, used as intended, and with basic precautions in place. I trust these routines because my health and my co-workers' depended on following them—and it worked.
Talk to anyone working with paints and coatings, and they’ll tell you that solvents make or break results. Dipropylene Glycol Propyl Ether, known in labs as DPGPE, isn’t some rare mystery fluid—its track record proves why it sticks around. In my years working on renovation crews, I watched painters breeze through tough jobs with products containing this glycol ether. DPGPE helps dissolve pigments and resins, preventing that pesky streaking or sticking. Spray paints keep their fine mist. Rollers leave fewer brush marks. That translates to faster drying, smoother finishes, and fewer callbacks about patchy walls.
People shopping for cleaning sprays or degreasers rarely check the label for DPGPE. Even fewer understand its impact. But those working in industrial cleaning or janitorial services spot its value fast. DPGPE cuts through oily, sticky residues without leaving streaks. I’ve seen maintenance teams tasked with everything from greasy restaurant kitchens to hospital floors reach for formulas containing this solvent. Its low odor means workers breathe easier, and its solvency power tackles tough grime. You want hospital surfaces disinfected, not just wiped off. Solvents like DPGPE give cleaners an edge over basic soap-and-water routines.
Print shops run on tight deadlines. Commercial printers rely on inks that won’t clog heads or dry too fast. DPGPE keeps ink flowing smoothly across substrates, boosting print clarity and color vibrancy. Over the years, talking shop with press operators made me realize how much the right solvent controls waste, cuts reprints, and keeps customer complaints low. In formulations for flexographic and gravure inks, DPGPE’s slow evaporation prevents nozzle blockages and ensures even drying on everything from newspapers to packaging films.
Washable clothes and crisp drapes owe some of their appeal to finishing chemicals. Factories use DPGPE in pre-treatment baths and finishing sprays for synthetic and blended fabrics. It dissolves additives that boost stain resistance or set dyes. Back in college, I worked a summer job at a textile mill and watched operators add DPGPE to tanks, then run endless bolts of fabric through. It smoothed out treatments and helped even application. Clothes came out of those baths softer, loaded with fewer leftover chemicals, and better able to hold color through countless launders.
A bottle of makeup remover, a can of hairspray, a gentle skin cleanser—each gets a boost from DPGPE. The solvent delivers active ingredients without irritating skin or overpowering a product’s scent. Chemists count on DPGPE’s ability to dissolve both water- and oil-based components, making it key in products aiming for gentle touch. The smooth, spreadable feel people expect can hinge on this one ingredient. My own favorite aftershave owes its cooling, easy-glide finish to the presence of glycol ethers like DPGPE.
Every industry loves a workhorse chemical, but none can ignore worker health or sustainability. DPGPE rates low for acute toxicity, but handling these solvents calls for common sense—and gloves. Companies shift toward water-based alternatives when possible, cutting solvent concentrations and exploring greener manufacturing. Strict training, real-time exposure monitoring, and improved ventilation keep employees safe, while ongoing research into safer substitutes backs up those efforts. Progress happens when chemists, managers, and regulators learn from real-world jobs and make choices that keep health and performance balanced.
Dipropylene Glycol Propyl Ether shows up in all sorts of products. Paint shops, cleaning supply warehouses, and plenty of manufacturing plants keep drums of this liquid on their shelves. Its reputation for blending well with water and oils makes it a favorite among manufacturers. But with popularity comes the responsibility to store it safely. I’ve seen what happens when folks overlook the basics—spills, ruined product, and unnecessary risks to people and property.
This chemical handles average temperatures fine, though it won’t last if exposed to extreme heat or blazing sunlight. I remember walking into a storage area on a humid August afternoon. Containers near the windows felt hot to touch, raising the chances for evaporation and fume buildup. Keeping containers in a cool, shaded spot lowers risks and saves money. I trust spaces with good airflow, since fumes don’t hang around and you don’t get that stuffy, loaded-with-chemicals feeling once you step inside. Over time, proper ventilation pays off by protecting workers’ lungs and keeping the air fresh.
I once saw a warehouse manager uncap a drum for “just a minute,” and that mistake cost several hours. This liquid picks up moisture and odors from whatever air drifts by. Letting any container stand open invites contamination. Workers benefit most by double-checking the seals after every pour. Labels, dates, and condition reports also keep everyone on the same page. If a drum leaks or a container warps, staff see it fast and can swap things out before contamination or accidents happen.
The liquid doesn’t burn as quickly as gasoline, though it still brings flammable risks. Workers need good training about keeping it away from sparks, static, and open flames. I’ve met teams who never mix storage of paint thinners, acids, or basic cleaners anywhere near Dipropylene Glycol Propyl Ether. This separation means, in case of a spill or leak, chemicals stay in their zones and don’t react poorly together. Simple spill kits near storage closets have prevented many headaches in my years on the job. Absorbent pads, gloves, and easy-to-read safety instructions give everyone confidence to handle a minor leak before it becomes a company-wide emergency.
Every plant, warehouse, or lab follows its playbook for safe storage, but government guidelines add another layer. The Environmental Protection Agency and OSHA spell out details for storing solvents and specialty chemicals. Clear pathways, readable Material Safety Data Sheets, and routine safety drills pull it all together. I always remind folks, policy can feel like a hassle, until it’s the lifeline in a high-stress moment. Gloves, goggles, eye-wash stations, and emergency showers should never collect dust.
Safe storage isn’t flashy, and rarely gets attention until something goes wrong. Experience tells me that paying attention to temperature, sealing containers, and clear labeling keeps everyone safer and keeps business running without surprise setbacks. Chemical safety grows from everyday habits, and Dipropylene Glycol Propyl Ether deserves that steady respect.
I’ve spent a few years working in labs and factories, and one lesson stands out: hidden hazards catch up with you fast. Dipropylene Glycol Propyl Ether, or DPGPE, often lands in cleaning products, paint thinners, and inks. It smells mild, almost sweet, so folks can overlook the fact that it’s a solvent with real bite, especially if you use it daily or slack off on safety. Inhaling the vapor or getting it on skin never feels threatening at first, but over time, headaches, dizziness, and skin irritation can set in. Anyone who’s cleaned up a spill without gloves learns quickly that solvent burns pack a punch.
Long shifts handling chemicals taught me that rushing or getting careless lands people in the emergency room. Respecting DPGPE from the start means suiting up properly. Nitrile gloves protect skin better than cheap latex, and a splash-proof apron helps during decanting or mixing. Cotton shirts and regular jeans don’t stop spills, so investing in chemical-resistant gear really saves trouble. Local exhaust ventilation, like a fume hood or at least a portable extraction fan, keeps the work zone clear, since breathing solvent vapor leaves techs groggy and forgetful.
Storing DPGPE in a tightly sealed container, in a spot with steady temperature and good airflow, also limits runaway vapors and cross-contamination. I always keep it away from acids and oxidizing agents—those reactions kick up heat and dangerous byproducts. Wiping up drips with absorbent pads beats using old rags, since even a small spill can spread quickly across a bench or floor, upping the slip-and-fall risk and making cleanup tougher.
No workplace gets through a year without a spill or two. The folks who prep and label spill kits always look like overachievers until you need them. Quickly isolating the area, popping on goggles, and clearing others out buys precious time. For a mess on a counter, I used to summon help first, then layer on absorbent pads—no mopping, which just pushes the mess around. Used cleaning materials go into a sealed bucket for hazardous waste pickup. If DPGPE gets on your skin, it pays to rinse under running water for a good 15 minutes, not just a splash and dash.
Many chemical handlers get basic training but then stall out. Laws require safety sheets and signs, but nothing replaces routine safety meetings and hands-on refreshers. I’ve seen near-mistakes drop off simply by practicing spill response every quarter and reviewing symptoms of exposure so everyone knows what to watch for. Even the pros miss signs sometimes—regular air quality checks with proper meters help catch vapor buildups early, long before someone gets woozy or clammy. Pairing safety culture with real follow-through keeps people healthy and on the job.
Dipropylene Glycol Propyl Ether plays a part in many industries, but it pays to treat it with the respect earned by any potent solvent. Upgrading protective wear, practicing smart storage, and running regular drills all drive down the risk and create a safer workspace. Having spent years in the trenches, I’ve seen how the right approach can turn a workplace from risky to reliable. That shift makes all the difference—not just for productivity, but for the people going home safe each night.
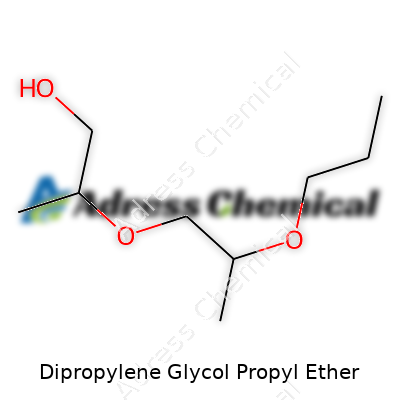
| Names | |
| Preferred IUPAC name | 1-(2-propoxy-1-methylethoxy)propan-2-ol |
| Other names |
DPnP Dipropylene glycol n-propyl ether Propoxypropanol Oxydipropanol propyl ether |
| Pronunciation | /daɪˌproʊˈpiːliːn ˈɡlaɪˌkɒl ˈproʊpəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 29911-27-1 |
| Beilstein Reference | 8812967 |
| ChEBI | CHEBI:82723 |
| ChEMBL | CHEMBL1377861 |
| ChemSpider | 191654 |
| DrugBank | DB14172 |
| ECHA InfoCard | 05ca70fd-78a4-4dd2-bf5f-20d5700d7391 |
| EC Number | 603-177-00-8 |
| Gmelin Reference | 1202711 |
| KEGG | C19582 |
| MeSH | D005899 |
| PubChem CID | 82132 |
| RTECS number | JM1575000 |
| UNII | R9U220V4P3 |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C9H20O3 |
| Molar mass | 206.32 g/mol |
| Appearance | Colorless liquid |
| Odor | Mild odor |
| Density | 0.949 g/cm3 |
| Solubility in water | miscible |
| log P | 0.93 |
| Vapor pressure | 0.129 mmHg at 25°C |
| Acidity (pKa) | ~14.8 |
| Basicity (pKb) | 7.43 |
| Magnetic susceptibility (χ) | -7.93×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.418 |
| Viscosity | 2.6 mPa·s (25 °C) |
| Dipole moment | 3.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 503.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -635.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4560.7 kJ/mol |
| Pharmacology | |
| ATC code | D01AE |
| Hazards | |
| Main hazards | May cause eye irritation. May cause respiratory tract irritation. May cause skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. |
| Precautionary statements | P210, P233, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 77°C |
| Autoignition temperature | 215 °C (419 °F) |
| Explosive limits | Explosive limits: 0.7% - 6.0% |
| Lethal dose or concentration | LD50 oral, rat: 3089 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral rat: 3089 mg/kg |
| NIOSH | 'NIOSH: TX2106000' |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Dipropylene Glycol Propyl Ether: Not established |
| REL (Recommended) | 0.1% |
| Related compounds | |
| Related compounds |
Dipropylene glycol n-butyl ether Dipropylene glycol methyl ether Dipropylene glycol dimethyl ether Propylene glycol methyl ether Tripropylene glycol methyl ether |