Chemistry advances because people keep finding better answers to tough problems. For decades, manufacturers searched for glycol ethers that gave just the right balance of performance and flexibility. Out of this steady push, Dipropylene Glycol Phenyl Ether emerged and found a natural place in automotive fluids, cleaners, and coatings by the 1980s. Production picked up after refiners of propylene oxide refined their own workflows and saw dipropylene glycol as a valuable fraction. The fine-tuning from lab to factory wasn’t instant. Going back over research reports or talking to people who worked in paint labs during the 1990s, it’s clear that many solvents tried to step into the big leagues, but very few stuck around. Dipropylene Glycol Phenyl Ether stayed relevant when others faded out simply because it met performance demands—no theories, just practical results.
Dipropylene Glycol Phenyl Ether stands out for how well it slides into a long list of uses. As a clear, nearly odorless liquid, it moves easily through pipelines and mixes naturally with ingredients in paints, printing inks, and industrial detergents. What speaks to me over the years is how suppliers rarely have to explain its benefits to formulators working on tough cleaning jobs or complex resin coatings—it backs up the promise in the real world. With customers looking for reliable supply chains and predictable formulations, this glycol ether often shows up as a backbone ingredient that professionals end up trusting after witnessing its track record.
This molecule has a moderate molecular weight and holds together at a boiling point approaching 290 °C. Unlike shorter chain ethers, it doesn’t flash off fast and can take a fair bit of heat. Water solubility remains low, which lets it serve as a bridging solvent in waterborne coatings but without dragging down film formation. With viscosity that feels neither too slick nor too thick, it pours and pumps reliably through pipes even on cold days in the warehouse. Over a decade of working with labs that test blends, it becomes obvious that this ether keeps stable in storage, which saves headaches for anyone looking at shelf-life problems. With a modest vapor pressure, workplace air keeps cleaner, meaning safety teams worry less during everyday use.
Every drum or container leaving a responsible plant keeps technical details front and center. The CAS number—6180-61-6—serves as a unique fingerprint for global trade, avoiding mishaps when large orders cross borders. Most suppliers guarantee purity above 98% to meet coating and cleaning market demands. Accurate labeling with batch numbers and hazard pictograms follows regulations to the letter, giving buyers what they need for compliance checks. Over the years, watching safety audits in big chemical warehouses, the value of an easily recognized label can’t be overstated. Lab managers want fast answers for traceability questions, and tight standards help prevent mix-ups.
Large-scale production doesn’t chase exotic chemistry. It starts with propylene oxide, forming dipropylene glycol through basic catalysis, then attaches a phenyl group through etherification. This route uses well-understood equipment, cutting down error risk and cost flare-ups. Batch reactors handle the process on a scale matching demand across cleaning and coating factories. I’ve seen plant operators praise modular setups that let them tweak ratios as orders shift. Their feedback made it clear: consistency counts more than any fancy-new approach that might promise fancy numbers in a paper but stops working in an actual plant.
This glycol ether boasts chemical stability in harsh environments, enduring acid and base without unraveling. Formulators blend it into recipes with chlorinated resins or reactive esters, counting on its inertia to preserve intended performance. It supports crosslinking in alkyd resin systems, plugs compatibility gaps, and acts as a reliable carrier—never stealing the spotlight, but always backing up the system. Once, a technical rep described it as “the straight man” in a double act—it doesn’t get the jokes, but helps everyone else land the punchline. The workhorses of industry find value in substances behaving predictably across cycles and temperatures.
Shopping around the world, Dipropylene Glycol Phenyl Ether answers to plenty of aliases—DPGPE, PPh, and phenoxypropanol among them. Big names in solvents offer it under proprietary brands, but the base chemistry stays the same. It streamlines sourcing for purchasing teams in multinational firms. In training new hires or walking through procurement checklists, the spread of names sometimes sparks confusion; that’s why documentation and open supplier communication turn from “nice to haves” into non-negotiable priorities. Standardized Safety Data Sheets (SDS) help avoid mishaps and clear those language gaps.
Handling glycol ethers safely means paying attention to every step, from delivery to cleanup. Health agencies weigh in on safe exposure, and companies that ignore these guidelines rarely last long. In the workplace, teams don gloves and goggles, keep local ventilation running strong, and store containers away from direct sunlight. I know EH&S professionals who stress the importance of spill kits and emergency eye washing stations. Experience proves that training new team members on protocols goes further toward accident prevention than relying on written signs alone. Workers who see steam or drips respond quickly when they’re prepared. Following OSHA and REACH rules protects both people and a company’s reputation.
Factories around the globe count on Dipropylene Glycol Phenyl Ether in cleaning chemicals, degreasers, and surface coatings. It cuts through stubborn grime where simpler alcohols fizzle out and provides solvency power for difficult pigments in paints and inks. Some textile and adhesive makers rely on its balanced drying time—slow enough to cover big surfaces, but not so slow it delays production lines. I’ve walked through plants where this solvent keeps dye flowing smoothly or brightens glass after only one pass with a washer. Years of feedback from industrial chemists speak to its ability to meet those tough, real-world challenges, not just the theoretical ones.
New regulations in every continent put pressure on solvent makers to find ingredients that perform without harming users or the environment. Researchers regularly test glycol ether alternatives, but Dipropylene Glycol Phenyl Ether keeps its spot because of its low acute toxicity and reliable function. I’ve read papers comparing it to more aggressive aromatics and saw those numbers come alive during pilot-plant runs—blends with this glycol ether outperform on gloss, open time, and minimal odor. Some forward-thinking labs focus on molecular tweaks, targeting even faster biodegradation and less environmental impact, but stay anchored in what the industry already knows is safe and proven.
Industry keeps a close eye on health findings for all glycol ethers. Dipropylene Glycol Phenyl Ether avoids the severe toxicity flagged in simpler glycol ethers used decades ago. Chronic exposure studies stack up favorably next to older solvents, with workplace limits set to levels science shows won’t harm users. Medical reviews and animal studies confirm that this ether doesn’t act as endocrine disruptor or carcinogen under realistic exposure. Modern facilities run periodic air monitoring and blood testing, not as a scare tactic, but as a way to keep up trust between workers and management. Over the years, both unions and company health offices recognize the benefit of continuing research, keeping warnings up-to-date and based on facts, not rumors.
Sustainability takes center stage in every industry, and Dipropylene Glycol Phenyl Ether faces the same scrutiny as any mainstream solvent. As customers demand more from what’s in their products, chemical producers invest in ways to lower emissions and push for cleaner manufacturing. Renewable feedstocks stand on the horizon, with research teams chasing routes that swap in sustainable raw materials without losing the predictable performance that made this ether a staple. End-users ask more questions, read labels more closely, and want proof that chemicals won’t create burdens for tomorrow. The companies that answer with transparency and innovation will guide the next stage—taking hard-earned lessons from sixty years of industrial chemistry and using them to set smarter standards for the decades ahead.
Dipropylene glycol phenyl ether steps into the world of modern manufacturing like an old workhorse few pay attention to, yet many depend on. You won’t hear much about it outside of technical papers or ingredient lists, but chances are it pokes its head into your day more than you realize. Known for its solvency and pleasant handling, this liquid goes into products people use and touch daily—cleaners, paints, inks, and personal care items just to start. Over time, the demand for solutions that blend performance with safer profiles keeps this compound relevant.
Household and industrial cleaners look for solvents that lift grease and dirt without hazardous side effects. Companies have moved away from old aggressive solvents that posed safety or environmental concerns. Dipropylene glycol phenyl ether brings powerful cleaning with a much better balance of performance and worker safety than some older petrochemical choices. For custodians scrubbing public schools or mechanics mopping oil from a shop floor, this solvent shows up in ready-to-use bottle formulations. It breaks down oily soils efficiently, rinses away more easily, and doesn’t kick up the harsh odors competitors might.
Walk through any hardware store, and see how many paint cans or ink cartridges line the shelves. Each claims vivid color, smooth mixing, and easy brushing. Formulators face the old problem: getting pigment to flow without clumping or drying in the nozzle. Dipropylene glycol phenyl ether acts as a co-solvent, reducing surface tension and keeping pigments suspended. Painters get longer open times on brushes and less effort scrubbing rollers after the job. Commercial printing shops use it to reduce clogging and get sharper runs in high-speed printing.
Shampoos, lotions, and even deodorants might contain small amounts of dipropylene glycol phenyl ether because it dissolves fragrances and active ingredients that don’t mix easily with water. Personal experience as a curious consumer led me to examine ingredient lists on everyday products. The goal is to deliver a pleasant experience in texture and scent, not just function. This solvent blends oils, extracts, and other additives smoothly so they don’t separate in the bottle or leave a sticky layer on skin.
Cleaner labels matter more every year. Consumers pay attention to how a product impacts both personal health and the environment. Factories look for chemicals that clear strict safety audits and do not require extreme measures for handling or disposal. Just because something dissolves well doesn’t mean it belongs in a family home or open waterways. Dipropylene glycol phenyl ether stands out because researchers have measured its low volatility, mild odor, and manageable toxicity profile. Regulatory reviews in regions like the US and EU keep potential risks in check. Substitute solvents sometimes fall short by evaporating too quickly, irritating skin or eyes, or producing strong fumes. Even in concentrated amounts, it’s less likely to trigger allergic reactions compared to other solvents.
Industry change will always be slow in areas tied to cost or tradition. Switching solvents often means reformulating products, retraining staff, and investing in new safety protocols. Yet, from experience in product development, innovation comes not from buzzwords but from stepwise improvements. Companies that keep searching for materials meeting higher human and environmental safety standards tend to earn trust. Dipropylene glycol phenyl ether isn’t a cure-all, but it represents a move away from harsh and hazardous ingredients. Keeping sight of worker safety, consumer health, and ecological impact sets the stage for the steady shift toward safer, more thoughtful chemistry.
Dipropylene Glycol Phenyl Ether often pops up in ingredient labels for cleansers, creams, and hair products. Chemists like it for its ability to dissolve other substances, cut through oil, and help blend scents smoothly into formulas. The material isn’t new, and it’s widely used across skincare, household products, and even industrial cleaners.
People caring about what they put on their skin have good reason to dig into ingredients. Several studies and government assessments have taken a close look at Dipropylene Glycol Phenyl Ether. The Cosmetic Ingredient Review (CIR) panel — a trusted independent safety committee with dermatologists and toxicologists — checked available safety data and didn’t spot major red flags for skin contact at concentrations used in personal care. Routine skin patch tests suggest a low risk of irritation or allergic response for most healthy adults.
In my own search for practical experiences, I checked with a few dermatologists. They pointed out that irritation risk climbs in people with broken or highly sensitive skin. Dry patches, eczema, or wounds may react to products containing synthetic solvents, including this one. So, while most people get through daily use without trouble, vulnerable skin types can show redness or discomfort.
Transparency in skin care means people know what they’re applying and can make choices that match their health needs. It gets hard to decide if a product is right without clear ingredient lists. With Dipropylene Glycol Phenyl Ether, labeling is usually straightforward, which helps. Sources like the European Commission’s CosIng database and U.S. Environmental Working Group assign low-to-moderate hazard scores, mainly flagging rare allergies. Both agencies call for more research on long-term exposure in higher concentrations, especially in sprays or airborne mists, where inhalation becomes possible.
If you’re careful with your skin, here’s what helps. Before switching to a new product, patch-test it on a small area inside the elbow or behind the ear. Wait 48 hours and watch for redness, bumps, or burning. If nothing happens, it’s likely fine for bigger areas. If you have eczema or persistent rashes, check labels and talk with a dermatologist. While most everyday lotions and shampoos use low doses far below alarm level, products for industrial or salon use can run stronger. Children tend to have more delicate skin, so milder formulas make sense for them.
Manufacturers can support safety by doing more than the bare minimum on testing, especially for leave-on skin care. Sharing results from new studies and updating safety data sheets builds trust. As more people become aware of ingredient sensitivities, there’s real value in including alternate products for those with extra-delicate skin.
Consumer groups and researchers push for fuller data on low-level, chronic exposure. Tracking products over time and sharing health impact findings will help regulators tighten safety standards where it counts. Transparency and science-backed choices empower people to care for their skin with fewer unknowns.
Walk down any household cleaning aisle and you’ll notice a wide selection of sprays, wipes, and degreasers. Many of these products rely on strong solvents to cut grease and lift grime. Dipropylene Glycol Phenyl Ether often finds its way into these bottles. This ingredient shines as a solvent because it mixes well with water and oils, and it helps dissolve sticky messes on countertops, glass, and even stovetops. Professional janitorial suppliers opt for it too, especially for industrial-strength cleaners used in offices or schools. By using an ingredient with a good safety record, companies navigate the balance between performance and workplace safety.
Anyone who’s painted a room—or watched paint dry on a factory assembly line—has witnessed the need for stable, reliable thinning agents. Dipropylene Glycol Phenyl Ether serves a practical role here. It improves the spread and flow of paints and varnishes. This keeps coatings smooth and helps them dry evenly. Printers and ink producers rely on it to stop ink from clumping or drying too quickly as it moves through industrial presses. Smudges, streaks, or dried-out nozzles spell trouble, so a versatile glycol ether reduces waste and helps produce clear results on paper, wood, or metal.
Shampoos, lotions, and deodorants often carry sweet or citrus scents, and behind those fragrances stands a host of carrier chemicals. Dipropylene Glycol Phenyl Ether acts as one such carrier. Companies use it to blend essential oils and dissolve active ingredients evenly across a product. It fits right into formulations and doesn’t leave behind a greasy feel. The rise of “free from” claims means formulators rely on ingredients that minimize skin irritation and allergic reactions. Years of toxicology research have built trust in this glycol ether for products used daily.
Manufacturing textiles and finishing leather calls for solvents that can help dyes and treatments stick well. Mills toss batches of fabric or hide into vats with chemical baths, and here, Dipropylene Glycol Phenyl Ether boosts absorption of dyes and finishes. Its chemical stability stands up to long processing times and hot conditions, reducing the chances of uneven colors or faded spots. I once toured a denim factory and saw piles of swatches tested for wash-fastness and color strength, with this compound listed on chemical manifests—a nod to how integral such ingredients have become for repeatable results.
Factories, hospitals, restaurants—these places run through gallons of cleaners and surface sanitizers every week. Surfaces range from stainless steel to plastic and glass. In these spaces, Dipropylene Glycol Phenyl Ether steps up as a heavy-duty cleaner that also limits residue and surface fogging. With stricter rules from OSHA and the EPA around workplace chemical exposure, suppliers look for solvents that perform but don’t push exposure limits. Hospitals especially pay attention to ingredient lists for any product used near food prep or patient care.
More research has started to focus on the long-term impact of industrial solvents, including their environmental fate. Alternatives with lower volatility and toxicity get a closer look from regulatory bodies and environmental groups. Committing to green chemistry means thinking about safer substitutes and making gradual shifts in manufacturing practices. The industries above shape their decisions based on worker safety, performance, and customer trust, and Dipropylene Glycol Phenyl Ether remains common because it checks a lot of those boxes.
Dipropylene Glycol Phenyl Ether sounds like a bit of a tongue twister. Folks in the chemical industry, cleaning supply business, and even cosmetics know this ingredient as DPPH or DPGPE. The chemical formula isn’t a random squiggle: it’s C15H24O3. That breaks down into fifteen carbon atoms, twenty-four hydrogen atoms, and three oxygen atoms. For people with chemistry backgrounds, this formula paints a quick picture. For everyone else, it’s a snapshot of how complex yet effective this molecule can be.
I once managed a janitorial supplies warehouse and noticed that many cleaning products listed ingredients like DPGPE right after water and surfactants. This molecule finds itself folded into floor cleaners, degreasers, and paints. Why does it show up so much? The science points to its blending skills—it can help dissolve both oil and water-based ingredients. This versatility lets product companies make formulas that cut through grease but don’t overpower with strong odors or leave sticky residues. In my own work, this meant less scrubbing and fewer complaints about streaky finishes.
Health organizations like the Environmental Protection Agency (EPA) and the European Chemicals Agency track DPGPE and its cousins for any dangers. Most safety sheets rate it as low in acute toxicity. If it lands on your skin or mists in the air, it usually won’t cause severe reactions unless someone is already sensitive. That said, any chemical in a concentrated form can irritate skin or eyes. Working with it in an industrial setting, I learned that gloves and basic ventilation were often enough to stay safe. Household users see very low amounts, so risks are much lower.
The EPA lists DPGPE in reports on chemicals found in water supplies and sewage. It doesn’t break down quickly if released in large amounts, so factories must watch how much escapes into drains. Regular water treatment usually catches the bulk before it goes far. Regulatory bodies demand strict reporting for big users, and manufacturers have moved toward tighter waste management practices. Companies got more transparent about ingredients after regulatory pushes in the early 2010s, and I saw product labels start listing dipropylene glycol phenyl ether by name rather than code numbers.
There’s a push to create greener versions or find alternatives with minimal environmental costs. Some companies switch out DPGPE for plant-based solvents, but it’s tough to balance eco-friendliness with the cleaning power customers expect. Research groups are testing new blends that stay effective but don’t linger in water supplies. Support for green chemistry has grown, and my colleagues who follow product development trends see an uptick in grant funding for safer, fast-degrading molecules.
Understanding the chemical formula of a compound like dipropylene glycol phenyl ether means more than memorizing numbers and letters. It’s about recognizing how behind every ingredient sitting on the shop shelf sits a web of safety decisions, technical facts, and environmental lessons learned. Industry experts, scientists, and health officials continue to review new data and adapt best practices as our understanding grows.
Spending years in the chemical business, I’ve seen how a little carelessness with liquid solvents can turn a busy day into a disaster. Dipropylene Glycol Phenyl Ether brings value to manufacturing, cleaning, and specialty products. In the wrong conditions, this clear solvent can pose health and safety risks most folks would rather avoid. Getting storage and handling right keeps workers safe, saves money, and meets the law. No one wants fines, but worse than that, no one wants people to get sick.
This ether won’t win awards for volatility, but that doesn’t mean a hot warehouse gets a free pass. Most suppliers I know recommend keeping it at room temperature, away from direct heat. Storing barrels near radiators or on hot asphalt leads to expansion, leakage, and stinky messes. Indoor storage makes sense, with shade and steady air flow. A simple thermometer on the wall can tell if something’s off. Believe me, you’ll notice the smell before it gets critical — so workers should know what to watch for.
I can’t count the number of times I’ve seen someone unseal a drum just for a quick transfer, then wander off, leaving it open “just for a minute.” That’s long enough to invite in moisture or spill valuable material. Sealed, labeled containers keep this solvent secure. Metal or plastic drums, with tight-fitting gaskets, give the best results. Labels must spell out the exact chemical name, hazards, and contact information. If a spill happens, you don’t want folks scrambling to guess what they’re cleaning up.
Chemical exposure creeps up quietly, and Dipropylene Glycol Phenyl Ether can irritate skin or cause headaches with long contact. Gloves, goggles, and long sleeves reduce accidents. I’ve watched stubborn folks ignore PPE, only to develop rashes or worse. No one gets points for “toughing it out.” Eye wash stations, gloves, and chemical-resistant aprons should always stay within reach.
Poor airflow builds up vapors that can make people sick, particularly in small mixing rooms or filling stations. Simple exhaust fans and well-placed vents reduce headaches and improve air quality. Bigger facilities often install monitors for unsafe concentrations, but smaller shops can usually solve issues with open windows or fans pointed outwards. Sometimes, a simple sniff test can catch a problem early — so encourage workers to speak up if they notice anything odd in the air.
This chemical’s flash point sits higher than gasoline, but don’t leave drums near open flames, sparking tools, or smoking areas. In one lab I worked, a quick slip near a space heater almost ended in a visit from the fire brigade. Grounding all metal containers, using non-sparking transfer tools, and a strict no-smoking policy near solvents keeps accidents at bay. Fire extinguishers and emergency showers nearby bring peace of mind and can save lives in a pinch.
Everyone trusts routine. Weekly inspections catch leaks, damaged drums, or faded labels. Spill clean-up kits should stay stocked and in plain sight. Employees need real, hands-on training with practice drills, not just safety posters in the break room. Even veterans benefit from reminders; tired habits are where most accidents start.
Safe storage and handling of Dipropylene Glycol Phenyl Ether call for discipline and respect. Companies that lock down these basics protect their staff, keep the environment clean, and avoid costly mistakes. With the right routines and some common sense, this chemical stays useful, not hazardous.
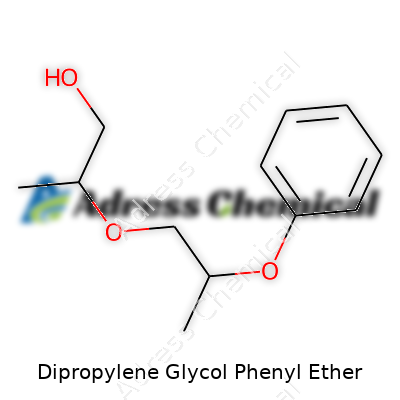
| Names | |
| Preferred IUPAC name | 2-[2-(Phenoxy)propoxy]propan-1-ol |
| Other names |
DPGPE Propylene Glycol Phenyl Ether Phenoxypropanol Phenyl Dipropylene Glycol Ether Oxyphenoxypropane |
| Pronunciation | /daɪˌproʊpiːliːn ˈɡlaɪˌkɒl ˈfiːnɪl ˈiːθər/ |
| Identifiers | |
| CAS Number | 6180-61-6 |
| Beilstein Reference | 2499454 |
| ChEBI | CHEBI:77737 |
| ChEMBL | CHEMBL3625392 |
| ChemSpider | 143834 |
| DrugBank | DB11286 |
| ECHA InfoCard | ECHA InfoCard: 100.140.944 |
| EC Number | EW-100-000-1 |
| Gmelin Reference | 607503 |
| KEGG | C18673 |
| MeSH | Dipropylene Glycol Phenyl Ether |
| PubChem CID | 82145 |
| RTECS number | JM1575000 |
| UNII | 4KO8R44L8M |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID0022062 |
| Properties | |
| Chemical formula | C15H22O2 |
| Molar mass | 242.32 g/mol |
| Appearance | Colorless liquid |
| Odor | Mild, faint odor |
| Density | 1.047 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.78 |
| Vapor pressure | 0.01 mmHg (20 °C) |
| Acidity (pKa) | 15.5 |
| Basicity (pKb) | 13.86 |
| Magnetic susceptibility (χ) | -7.48×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.497 |
| Viscosity | 130 cP (25°C) |
| Dipole moment | 3.63 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 418.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -619.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6530.6 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P333+P313, P363, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 110°C |
| Autoignition temperature | 300°C |
| Lethal dose or concentration | LD50 (Oral, Rat): 3,000 mg/kg |
| LD50 (median dose) | 2,600 mg/kg (rat, oral) |
| NIOSH | Not established |
| REL (Recommended) | 0.1% |
| Related compounds | |
| Related compounds |
Propylene glycol methyl ether Dipropylene glycol methyl ether Propylene glycol phenyl ether Tripropylene glycol methyl ether Ethylene glycol phenyl ether |