If you spend much time around industrial chemistry, you cross paths with Dipropylene Glycol Methyl Ether, better known in labs and warehouses as DPM or DPGME. The chemistry trade started using glycol ethers in the early twentieth century, drawn to their ability to mix oil and water and dissolve tricky substances. The methyl derivative trailed not long behind, picked up by the paint and coatings sector looking to cut down on noxious smells without sacrificing performance. DPM hooks into that chain: a by-product of propylene oxide reactions, matured through decades of refinement to serve as more than just a solvent. In the push for safer workplaces in the 1970s and 1980s, companies wanted less toxic alternatives to old-school glycol ethers, and DPM came through as an answer that balanced low vapor pressure and a lower hazard profile.
DPM belongs in the family of glycol ethers, a large clan with dozens of siblings, each with small tweaks that send them into wildly different uses. Despite sharing core chemistry, DPM’s ability to balance volatility and solvency sets it apart. You’ll find it in cleaners, coatings, printing ink, and even in perfumes. The product usually appears as a clear, nearly colorless liquid with a mild odor—hardly offensive, almost forgettable, which is a comfort when working around drums of the stuff.
Ask anyone working a QA bench, and they’ll rattle off the properties: DPM boils around 190–194°C and weighs in around 0.95 grams per cubic centimeter. It stays stable under regular conditions and doesn’t catch fire easily—flash point usually measured higher than many common solvents (about 75°C). It dissolves polar and nonpolar substances—rare for an organic solvent. Water blends with it in any ratio, and oil-based substances surrender almost as easily. For the everyday user, this means one tool cleans a wide range of messes and mixes well with most working formulations.
Chemists and buyers appreciate data. Standard technical sheets place purity around 95% or more, with water content typically kept below 0.1%. Reputable suppliers include chromatograms and distillation curves, proof that off-spec material won’t foul up a product run. For shipping and storage, UN numbers and pictograms warn handlers about risks; DPM rarely classifies as hazardous for transport, which smooths logistics across borders. Still, folks pay attention to flammability and proper ventilation warnings on every drum or pail.
If you peek into manufacturing, you’ll find DPM born from the reaction of propylene oxide with methanol, typically in the presence of a catalyst. It’s less glamorous than a lab synthesis—reactors churn away batch after batch, with distillation columns separating out the desired product from assorted isomers and side-products. Efficiency matters, and industry discoveries about reaction conditions or improved catalysts feed directly into lower costs and less waste down the line.
For chemical engineers, DPM stands as a building block as much as a solvent. The ether linkage resists attack but can be cracked under forceful conditions. Its —OH group on the glycol branch opens doors to further etherification or esterification. Add a strong acid or base, and the molecule acts predictably—a big reason why folks relying on batch-to-batch consistency stick with it for years. Formulators sometimes tweak it further, building in other functionality for custom cleaners or specialty inks.
Anyone in procurement or compliance wonders if DPM goes under another cloak: Dipropylene Glycol Monomethyl Ether, Propylene Glycol Methyl Ether D, or the ever-beloved CAS number 34590-94-8. Global buyers quickly memorize these descriptors. Every catalog, from Sigma-Aldrich to regional chemical houses, lists a handful of synonyms to stave off regulatory confusion. One bottle on the shelf might show DPM, another DPGME, but the liquid inside stays the same.
Operators who’ve worked with rougher solvents appreciate DPM’s friendlier profile, but nobody lets their guard down. The liquid can irritate the eyes and skin on direct contact, and inhalation of mist finds its way to headaches or lightheadedness after a long shift. Regulatory limits, like ACGIH’s threshold limit value of 100 ppm, push folks to ventilate workspaces and wear goggles or gloves. Spills seldom result in disaster, but nobody wants a sticky floor or long-term exposure. Waste management lands in the non-hazardous camp in many jurisdictions, keeping disposal costs reasonable, but documentation and tracking don’t get skipped.
Daily, DPM slips into a surprising array of products. The coatings industry loves its slow evaporation, which means paint dries evenly and leaves fewer brush marks. Cleaners—especially heavy-duty ones and degreasers—lean on DPM to cut oily residue from factory floors or kitchen tiles. Ink manufacturers value its solubility and ability to keep pigments dispersed. Even cosmetics and fragrances count on DPM’s mild nature and ability to carry both waterborne and oil-based actives. Its low odor lets it handle scent-heavy jobs without interfering with final fragrance notes.
Scientists and product developers keep looking for solvents that clean as well or better but aren’t likely to lead to headaches or long-term health issues. Research lately focuses on greener manufacturing—catalysts that run cooler, processes that cut waste, and reducing by-products. Analytical teams test batches for impurities, hunting for anything that might trigger regulatory headaches or consumer complaints. Some labs explore blending DPM with other glycol ethers to tweak evaporation rates for specialty uses; these efforts filter down over years as improved recipes and safer alternatives in end-use products.
Anyone seeing a material safety data sheet for the first time breathes a sigh of relief at DPM’s relatively low acute toxicity compared to traditional glycol ethers. Workers exposed for short periods rarely report symptoms if they follow basic safety rules. Chronic studies dig deeper—testing for liver impacts, reproductive health, and skin sensitization. So far, research groups haven’t linked DPM to serious long-term health effects under normal workplace exposure. Still, regulators recommend caution, keeping occupational limits conservative and watching ongoing animal and cellular studies for new findings.
Looking around the corner, DPM won’t disappear from formulation tables any time soon. Changes on the horizon come from the push for sustainability and changes in regulatory climate. Producers look for cleaner production methods, with low-energy, high-yield routes taking center stage across the chemical sector. Environmental concerns drive research into faster biodegradation and potential replacements from renewable feedstocks. For now, routine tasks in paints, inks, and cleaning products keep DPM a staple, but smart companies keep one eye open for shifts that could bring safer, greener chemicals with similar perks.
Most folks never give Dipropylene Glycol Methyl Ether a thought, but this chemical quietly shapes a chunk of what makes houses feel clean and paint go on smooth. You see it everywhere once you know what you’re looking for. At home, cleaning sprays and glass cleaners work so well because of this solvent. It breaks down streaks and grime so a paper towel can take it away. Most glass cleaning products in my cleaning cabinet list this on their labels because it can fight fingerprints but won’t leave a fog after wiping. That’s a big deal—nobody likes seeing residue after trying to clean the window in the afternoon sun.
Walk down any paint aisle, and there’s a good chance the bright, even finishes owe their look to Dipropylene Glycol Methyl Ether. Paint companies use this chemical to stop paint from drying too fast. If you’ve ever tried painting a hot wall and found that the paint dried before you finished a stroke, you’ll know why this matters. The solvent gives painters a better chance at getting a smooth finish—not just on walls, but on cars and cabinets too. Real world experience? My own garage door has held its color and smooth finish for a decade, largely thanks to coatings built on solvents like this one. Factories turn to it for making water-based paints that perform like old-school oil paints but without the strong smell or high toxicity.
Every workbench or industrial floor sees its fair share of oil and sludge. Businesses need strong degreasers that don’t force workers to wear heavy-duty respirators every day. Chemical engineers picked Dipropylene Glycol Methyl Ether for industrial degreasers and cleaners exactly because it has the power to cut through grease without burning nostrils or skin. These degreasers show up in auto repair shops and heavy equipment garages, lifting off machine oil so techs aren’t scrubbing for hours. I’ve used these at home for cleaning up a stubborn barbecue grill or greasy engine parts—the difference before and after shows just how much punch one chemical can have in regular life.
Diving into the print world, the story’s the same: printers need inks that don’t streak or dry up inside the nozzle. Print shops have told me that inkjet ink with Dipropylene Glycol Methyl Ether lets customer posters dry fast on paper but doesn’t gunk up machines. Bright magazine covers and product labels owe part of their sharpness and durability to this one ingredient. Nobody wants to shell out money for a new printer every few months or deal with lines across their photos—this chemical helps avoid all that downtime.
For all these uses, safety matters just as much as performance. Even though Dipropylene Glycol Methyl Ether ranks lower on the hazard scale than many older solvents, companies still treat it with caution. Any chemical that gets into home cleaners or factory floors needs routines for storage, proper labeling, and waste management. Workers handle it with gloves or eye protection; I always open a window if using a product at home with this ingredient. Regulators in places like the United States and Europe set rules on exposure limits. It’s smart—no point in fixing one problem while causing another.
Green chemistry is changing the conversation. People want cleaning power and paint quality without a long list of warnings. Academic labs and startup chemists are busy designing new chemicals or tweaked versions that keep the good parts—solvency, slow drying, no strong smell—while lowering risks even more. Some groups are already mixing plant-based ingredients into the same products, hoping to shed reliance on fossil fuel derivatives. It isn’t just about what works; it’s about what keeps homes, hands, and the air a little safer for the long haul.
Dipropylene glycol methyl ether finds its way into all sorts of products — window cleaners, paints, inks, even some cosmetics and air fresheners. Industry likes it for its ability to dissolve grease and mix with water. Anyone who has tried to remove sticker residue from glass with a spray cleaner may already have rubbed it into their skin or inhaled a little, without knowing what it was.
At home or at work, skin comes in contact with this solvent mostly through cleaners or by breathing vapors. The U.S. Environmental Protection Agency and the European Chemicals Agency both have checked out its profile. They say low concentrations cause little trouble, with no special rules telling people to avoid it in everyday household products. My own experience scrubbing countertops and mixing paints has proven that gloves can help, since strong cleaners tend to dry out or irritate the skin, whether it’s because of this solvent or something else in the mix.
Dipropylene glycol methyl ether isn’t the kind of chemical that sets off big alarm bells like formaldehyde or benzene. Short interactions rarely trigger serious reactions, which makes sense given its wide approval for use. Intense, repeated exposure, such as in factories or workshops, can lead to headaches, some eye or nose stinging, maybe dryness on your hands. Folks working in large-scale manufacturing sometimes report feeling a bit woozy after hours spent in confined, unventilated rooms. In that sense, it’s much like a lot of everyday solvents — safe in small doses, not so pleasant if you forget to open the window or leave a bottle uncapped for hours.
Healthy adults aren’t the most vulnerable group. Children with asthma and people who react easily to chemicals have more to gain by avoiding direct or concentrated exposure. Sometimes, spray cleaning in a small bathroom with the door shut leaves lingering odors or a tickle in the throat; anyone with strong sensitivity will want to crack open a window or step out for five minutes. Pregnant workers and those already struggling with chemical sensitivities might need stricter attention.
Precaution never hurts. Wearing gloves, adding ventilation, not mixing chemicals, and reading product labels all play a role. I remember working as a janitor after college: the day I decided to finally wear thick nitrile gloves, my hands stayed less chapped, and my headaches faded. It’s easy to forget that habits — like washing up after using strong cleaners — can build up a decent layer of safety without expensive gear.
Regulators suggest keeping workplace air levels below certain thresholds. For homes, airing out the kitchen or bathroom stops you from breathing in more than needed. Most people won’t run into dangerous amounts just cleaning counters or mopping floors, but being aware, reading labels, and taking those simple steps matter, especially if something feels off.
Companies could help more by listing these ingredients plainly or offering safety advice on packaging. That small step could cut down confusion and make it easier for everyone to handle chemicals safely, no matter their background or health.
Anyone who’s worked with cleaning products, paints, or even electronics has probably crossed paths with something called Dipropylene Glycol Methyl Ether. In science talk, folks often refer to it as DPGME. It bears a chemical formula of C7H16O3 and you’ll find its CAS number is 34590-94-8. This isn’t the sort of fact you hear around a coffee table, but it’s the bread and butter for workers and manufacturers who handle chemicals every day.
My first warehouse gig ended up teaching me more chemistry than I expected. Pallets and barrels filled with stuff like DPGME come with paperwork you can’t ignore. Not all chemicals print their risks clearly across the label, so everyone relies on these CAS numbers to make sure nothing gets mixed up. One number, one substance: that’s the way to dodge trouble when handling hundreds of compounds.
Dipropylene Glycol Methyl Ether acts quietly, too. With its mild smell and moderate vapour pressure, it blends pretty easily into all sorts of applications, from degreasers to ink solutions. I remember getting schooled by a maintenance guy, who said, “If you want paint that goes on smooth and dries before lunch, you add stuff like this.” Turns out, he was right. Products made with DPGME end up cleaner, less streaky, and easier to handle.
In an industry setting, knowing a formula like C7H16O3 gives folks a way to figure out how safe they’ll be, how far you can push the product, and what to expect if it makes contact with the wrong stuff. The CAS number, on the other hand, makes the paperwork trail clear. I've filled out safety data sheets, hazard placards, shipping manifests. If you write the wrong number, you set the stage for confusion, accidents, or even fines during a surprise inspection.
Beyond labeling, there’s a safety angle to all this. When someone calls about a chemical leak, emergency responders check their lists. No one wants a firefighter running into a situation blind. They look for that unique CAS number: 34590-94-8 tells them exactly what they're up against—so they can break out the right protective gear and know how to handle disposal.
Overexposure to DPGME has its risks: dizziness, headaches, maybe worse after long hours. I’ve seen old-timers who stopped wearing gloves and later complained about dry or itchy hands. That kind of daily exposure stacks up. Plus, with regulation tightening, bosses can’t ignore safety protocols. MSDS sheets land in every breakroom, and training gets real attention—not just a box ticked every quarter.
In my experience, a clear approach works: label every drum with the right CAS number, keep ventilation running where folks use this solvent, and make gloves and goggles routine. Over the years, tech has helped a bit, with better air monitors and tracking. But nothing beats a culture where people take these details seriously. Solid habits don’t leave much room for “I didn’t know.”
Working with chemicals, there’s no skipping steps. The chemical formula tells you what’s inside. The CAS number keeps everyone on the same page. Lessons learned from warehouses, print shops, and back rooms show that losing track of either detail can set off a chain of avoidable problems. Label well, stay informed, and remember—knowing your DPGME from the next solvent makes all the difference for safety, product quality, and everyone’s peace of mind.
Dipropylene Glycol Methyl Ether (DPGME) shows up in all sorts of manufacturing settings—paint shops, ink mixers, cleaners, and labs. Anybody who’s spent hours in the service bays or around big mixing vats has probably caught a whiff or two of the stuff. Left in the wrong spot or handled carelessly, it can put a lot more than your nose at risk. Spills and fumes can mess with your lungs, irritate your skin, or, if you knock over a barrel, make for one expensive cleanup day. Those who’ve worked around solvents learn fast—disorder becomes danger before you can blink.
Veteran workers rarely put chemical drums near busy walkways or under direct sun. Solvents like DPGME don’t fare well in the heat. Hot spots mean swelling containers, sticky leaks, or even fire if there’s enough vapor and a rogue spark. Steel cabinets and drum lockers, tucked away from direct light and heater vents, help keep the stuff cool. More than once, I’ve seen improvised storage—old shelves, stacked pails, boxes half-covered in dust—turn into a headache for everyone around. This chemical asks for a solid, ventilated storage spot, away from anything that burns easily, with the cap screwed tight and the drum upright. Old timers swear by double-checking those seals after every use. I do, too—I once left a lid loose, and lost half a shift cleaning up the slow drip that followed.
I once saw a breakroom right next to a solvent cage; lunch got a lot less appetizing when someone spilled near the door. DPGME shouldn’t get anywhere near food or drinking water. A locked door, a clear sign, and never storing solvents in old food containers keeps things glaringly obvious. Mixing up bottles is an accident in the making, so anyone with sore hands or foggy goggles needs clear, readable labels that won’t rub off the moment you touch them.
If you’ve worked with DPGME for a while, you’ll recognize what happens when safety rules go ignored—itchy eyes, sore noses, and headaches that kick in just a few minutes later. Proper gloves make all the difference; not every cheap pair from the discount aisle holds up against chemical spills. Nitrile offers more protection than thin latex, so it pays to check manufacturer details. Eye protection isn’t optional; splashes are sneaky and hard to see. People might roll their eyes at the “always wear your specs” posters taped above every sink, but there’s a reason those reminders never leave the wall.
That sharp, almost sweet smell? DPGME on the move. Without a proper exhaust hood or window cracked open, fumes hang low and build up. If you’ve caught yourself coughing after pouring a few liters, chances are the air’s already too thick. Running fans and vent systems not only makes the shifts less stuffy but keeps the exposure well under legal limits.
In the real world, containers tip or leak no matter how carefully they get stacked. Absorbent pads or spill kits should always be within arm’s reach; the worst spills happen about five minutes after the cleanup gear gets shoved in a corner. Once, a rookie kicked over a jug, hesitated, and let the puddle creep under half the workbench. We ripped open the spill kit with seconds to spare. Better to overprepare than mop chemicals with floor rags, risking skin and breath.
Rules aren’t enough. In every size shop, just a few well-run training sessions go a long way. Everyone benefits from hands-on practice—tightening lids, reading labels, finding the nearest vent switch or spill kit. Real safety comes from habit, not handbooks. If people understand what’s at stake, they start looking after themselves and their coworkers, and bad spills or burns become a rare story, not an everyday scare.
Dipropylene Glycol Methyl Ether goes by a few names—DPGME, for short. So, what's it really like up close? Pour some out in a beaker and you’ll see a clear, nearly colorless liquid. It doesn’t give off a strong smell; just a faint, somewhat sweet whiff. Its consistency reminds me of cooking oil: not runny like water but not sticky either. This stuff doesn’t evaporate right away—the slow pace says a lot about how it behaves in a workspace.
DPGME starts boiling at about 190 to 198°C. Most paints and cleaning products run into trouble if you pick a solvent that flashes off too fast. DPGME sticks around, giving coatings time to spread out evenly and settle. Turn the thermometer the other way, and you’ll see it won’t freeze until the temperature plummets past -80°C. Whether in a chilly warehouse or a humid storeroom, DPGME doesn’t turn solid or get sluggish. That comes in handy if your product needs shelf stability through all seasons.
Plenty of factory work teaches you to watch what happens when chemicals mix. DPGME blends with water, alcohols, and other glycols without fuss. No layers form, no gelling up. In my time helping formulate industrial cleaners, there’s always that moment you add the solvent and cross your fingers, hoping things don't separate. With DPGME, it just disappears into the batch, making it easy to keep a formula simple and reliable.
The density clocks in at about 0.95 grams per cubic centimeter, which feels just a touch lighter than water as you pour it. This helps when loading drums and measuring doses in a plant—you’re not wrangling something that splashes or sloshes everywhere. Its viscosity hovers around 4 to 6 centipoise at room temperature. For those unfamiliar: think thicker than water, but not as heavy as honey. In spraying and cleaning, this hint of thickness stops drips and runs. You get a controlled application.
Safety rules matter. DPGME's flash point sits at about 75°C, much higher than many other solvents. That means it won’t ignite unless things heat up beyond what any normal process would hit. I've dealt with solvents you can light with a match at room temperature—not the case here. For folks worried about storage or workplace safety, it's a real comfort.
Solvents that vanish into thin air cause headaches—literally and figuratively. DPGME’s vapor pressure is low, around 0.29 mm Hg at 20°C. In years spent in maintenance shops, low vapor pressure means less odor lingering in the air and lower risk of indoor air pollution. Floors and machinery don’t dry out too fast so you get time to work before things evaporate.
Quality matters in production jobs, whether you’re making paint, cleaning solutions, or inks. DPGME’s qualities—slow evaporation, easy mixing, steady handling—cut down on waste and mistakes. You won’t end up scrubbing equipment or redoing a job because the solvent dried out quicker than expected. Standards in manufacturing keep getting stricter, and DPGME is one of those tools that quietly makes a difference without drama.
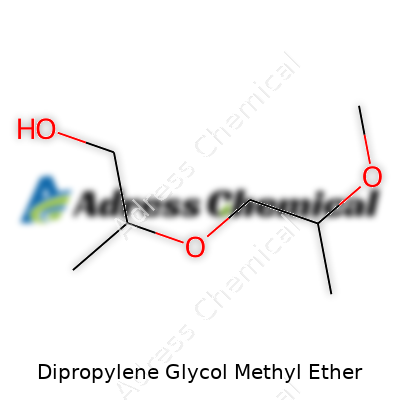
| Names | |
| Preferred IUPAC name | 2-(2-methoxypropoxy)propan-1-ol |
| Other names |
DPM 1-(2-Methoxy-1-methylethoxy)-2-propanol Dowanol DPM Propylene glycol methyl ether acetate Methoxypropanol Di(propylene glycol) methyl ether |
| Pronunciation | /daɪˈproʊpiːliːn ˈɡlaɪkɒl ˈmɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 34590-94-8 |
| Beilstein Reference | 1207955 |
| ChEBI | CHEBI:82858 |
| ChEMBL | CHEMBL2106728 |
| ChemSpider | 15357 |
| DrugBank | DB14006 |
| ECHA InfoCard | 03e92ec2-6f1b-4f1b-89f8-17bbc487ec02 |
| EC Number | 34590-94-8 |
| Gmelin Reference | 1620 |
| KEGG | C14326 |
| MeSH | D006824 |
| PubChem CID | 8147 |
| RTECS number | JM1575000 |
| UNII | K67R9E3D5S |
| UN number | UN2325 |
| CompTox Dashboard (EPA) | DTXSID9020939 |
| Properties | |
| Chemical formula | C7H16O3 |
| Molar mass | 134.20 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Mild, ether-like |
| Density | 0.951 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.56 |
| Vapor pressure | 0.03 mmHg @ 20°C |
| Acidity (pKa) | ~14.8 |
| Basicity (pKb) | 7.43 |
| Magnetic susceptibility (χ) | −32.8 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.404 |
| Viscosity | 1.0 – 2.0 cP (25°C) |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -634.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4591 kJ/mol |
| Pharmacology | |
| ATC code | D02AX |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P304+P340, P312, P337+P313, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 84°C |
| Autoignition temperature | 190 °C |
| Explosive limits | 1.1% - 14% (in air) |
| Lethal dose or concentration | LD50 (oral, rat): 5,134 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5,350 mg/kg |
| NIOSH | WIth the information you provided, here is the string for the NIOSH of Dipropylene Glycol Methyl Ether: "UUID795R651 |
| PEL (Permissible) | 100 ppm (TWA) |
| REL (Recommended) | 10 ppm |
| Related compounds | |
| Related compounds |
Diethylene Glycol Methyl Ether Propylene Glycol Methyl Ether Tripropylene Glycol Methyl Ether |