Back in the late 20th century, chemical manufacturers started looking for alternatives to old-school solvents, chasing both better performance and safety. Dipropylene glycol methyl ether acetate—often called DPM Acetate, DPGMEA, or by its mouthful of an IUPAC name, 1-(2-Methoxy-1-methylethoxy)-2-propanol acetate—showed up as a reliable solution. Over a few decades, it made its way from lab research to the buckets and barrels in factories across coatings, paints, printing, and electronics. Big players in Asia and Europe invested in capacity as demand grew, while smaller outfits tracked these giants to supply regional markets. Trade names like Dow’s DOWANOL DPM Acetate show up on global spec sheets, making this chemical something you’re likely to encounter if you spend any time around solvents.
DPM Acetate serves as a solvent mainly, used in cleaning products, lacquers, and certain inks. A clear, nearly odorless liquid, it brings a unique set of skills: low volatility, balanced solvency, a relatively high flash point. That means less evaporation, better safety when handling, and a smooth job dissolving resins and polymers in product formulations. Typical specs list purity around 98–99%, with water content as low as possible. The material’s chemical makeup—a glycol ether acetate—lets it work where harsh traditional solvents simply can’t go. Its use cuts down the sharp smells and health complaints that used to follow paint jobs in closed rooms or industrial halls.
Pour DPM Acetate into a glass and you’re looking at a liquid with a boiling point around 190°C, a flash point above 75°C, and density about 0.96 g/cm³. These figures barely move between batches from reputable makers. Labels on drums show UN numbers, storage warnings, and the familiar “flammable” symbol, though less alarming than what sits on pure acetone. Cas Number 88917-22-0 sits on technical data sheets. In real-world use, good ventilation helps manage exposure, and nitrile gloves protect against the slow absorption small spills can cause. Good labeling isn’t only about legal compliance—it pushes safer handling and fewer headaches for workers.
To get DPM Acetate, manufacturers start with dipropylene glycol methyl ether, react it with acetic anhydride or acetic acid, and run the mix through catalytic processes. Much of this chemistry takes place in closed reactors at industrial scale, monitored to keep byproducts just about nil. With every tweak of pressure and temperature, chemical engineers chase better yields and cleaner product streams. The equipment sets the tone: stainless steel, inert linings, and constant quality checks. The acetate group really makes a difference, plugging into the molecule where it tunes both volatility and solvency.
DPM Acetate holds up against hydrolysis under most working conditions, but strong acids or alkaline washes will break it back down into the parent glycol ether and acetic acid. That reactivity comes in handy for certain industrial cleaning and stripping processes. Outside of that, it stays relatively stable, even under light and moderate heat. Some material scientists play with chemical modifications, building off the ether backbone to chase new additives or functional solvents. Over time, these experiments may open new doors for DPM Acetate in unfamiliar sectors.
It’s easy to get tripped up among synonyms: Propylene glycol methyl ether acetate, DPM Acetate, PGMEA (though that last one often means a related but different chemical), Solvenon DPM Acetate, and a string of numbers—CAS 88917-22-0, EC 500-237-8. One trip through a supplier catalog brings up half a dozen trade names, depending on region or batch specifics. While confusing, these different identifiers let buyers match specs tightly across industries, cutting risk and confusion in large procurement runs.
DPM Acetate doesn’t bring the same lung-burning bite or fire risk as old-school acetates, but it still calls for respect. It can cause skin and eye irritation, so most facilities lean toward gloves, goggles, and well-ventilated areas. MSDS (Material Safety Data Sheets) flag its hazards, clearly highlighting routes of exposure and emergency procedures. Employers put up poster-size labels in mixing rooms, run regular spill drills, and document safe handling. Regulatory standards—set through agencies like OSHA and the EU’s REACH—keep tightening these guidelines, trimming exposure limits as new research comes in. Routine monitoring and smarter storage practices—away from strong oxidizers and open flames—play a big part in everyday safety.
Nearly every industrial paint, from automotive to marine coatings, leans on DPM Acetate as a core ingredient. It dissolves pigment and resin so that application goes on smooth, without streaks or unpredictable drying times. Printers—especially those doing high-end digital and offset jobs—use it to keep inks in just the right state for drying on glossy paper. In the electronics sector, it cleans delicate circuit boards without leaving residues that jam microscopic connections. Even some cleaning products hide it on their labels as a solvent that unlocks stubborn stains without clouding up hard surfaces. Some personal experience with shop floors and paint booths taught me how versatile it could be, often swooping in to replace two or more more dangerous solvents on the shelf. Across these sectors, the chemical’s ease of blending and safer profile keeps it in production lines even as standards get stricter.
Labs and manufacturers both spend good money testing how DPM Acetate interacts with the next generation of inks, plastics, and composite materials. Cutting-edge research ties it into organic electronics and flexible displays, places where solvent purity and residue levels make or break results. Environmental chemists poke at its degradation products, hoping for an ecological win. Formulators eyeing “green” certification know that every solvent molecule counts, so the pressure stays on to either improve DPM Acetate’s renewability or swap it out for a biobased cousin. In start-up digests and R&D reports, you’ll find experiments where enzyme-based routes pump out glycol ethers from plant sugars, then swap them through acetylation for properties nearly indistinguishable from fossil-fuel origins.
DPM Acetate rates as moderately hazardous—less threatening than some glycol ethers, but certainly not harmless. Researchers track acute toxicity through inhalation, skin contact, or accidental ingestion; most published work notes mild irritation above threshold levels, with organ toxicity only present at absurdly high exposure rates in rodents. Occupational medicine teams still debate whether chronic low-level exposure raises any cancer risk; long-term animal studies haven’t turned up clear links, but regulatory agencies push for periodic reviews as new epidemiological data rolls in. Many production facilities have responded by running tighter air monitoring and breakroom education sessions. Familiar worksite lessons—wash hands, wear proper gloves, don’t take lunch in the chemical area—do more than dry pronouncements from a lab textbook.
The next ten years may see a shift, as industries double down on sustainability targets. Manufacturers work to reduce atmospheric emissions during production through tighter seals, solvent recovery, and process recycling. Life-cycle analyses now accompany technical datasheets, as buyers ask hard questions about raw materials, waste, and downstream environmental impact. Engineers keep eyeing ways to cut the carbon footprint, perhaps by moving toward bio-derivatives or closing resource loops in manufacturing hubs. All of this matters as stricter import regulations and consumer scrutiny guide chemical choices on global markets.
A lot of folks might not spend time thinking about industrial solvents, but Dipropylene Glycol Methyl Ether Acetate—often shortened to DPMA—shows up inside products more often than you'd expect. Take a stroll down the paint aisle or step inside a repair shop, and you’ve shaken hands with DPMA. This chemical gets tucked into paints and coatings because it helps pigments spread smoothly and keeps things level. Proper spreading matters. Nothing frustrates like painting a wall only to end up with splotches and streaks.
DPMA doesn’t just help with nice-looking paint—it gives workers more time to work. Its slow evaporation lets you take a breath, make adjustments, and avoid the panic of a drying roller. Construction crews and artists alike benefit from that window of flexibility. Companies favor DPMA in industrial floor coatings and car finishes, since reliable performance matters far more than just making things look pretty.
Many inks in printing houses rely on DPMA because it doesn’t evaporate too suddenly under heat. When printing runs hit high speeds, keeping the ink from gumming up the machinery is critical. Consistent performance helps press operators avoid costly downtime. Magazine covers, packaging, and product labels owe much of their smooth graphics to DPMA’s presence in ink formulas.
Wide-format printing also thrives here. Without dry or brittle spots, billboards and banners come out cleaner and last longer in the elements. For illustration and signage printers, stable ink means less waste. Every extra sheet or print they save means money back in their pocket—in an industry with tight margins, that matters.
Get into a gadget teardown and you’ll find circuit boards covered in protective coatings. DPMA helps make it possible. It slots into electronics cleaning agents and coatings because it dissolves grime and flux residues without eating away at sensitive components. In my early years fixing computers, I relied on products filled with DPMA to erase stubborn soldering residue. Without those chemicals, repairs would have left behind corrosive bits that shorten gadget lifespan.
Touchscreens, circuit boards, and wiring enclosures all lean on solvents with the right properties—ones that clear off residue but leave no trace themselves.
No chemical should get a free pass on safety. DPMA brings a lower vapor pressure and reduced odor compared with harsher solvents once common in factories. Health concerns don’t disappear, but the switch to DPMA has made life simpler for workers in confined spaces or those sensitive to fumes. Protective gear and proper ventilation still remain part of daily life, but moving away from solvents like toluene or xylene is a win.
Wastewater treatment facilities have to grapple with what happens down the line. Research from the last decade describes how DPMA breaks down more easily than some old-school solvents, making it just a bit less of a headache for folks cleaning the water supply. Eco-friendliness remains a tricky balance—it serves in places where water-based solutions still fall flat.
There’s room for better answers as green chemistry grows. Scientists test new blends of plant-sourced solvents, aiming for lower toxicity and less environmental fallout. The delicate balance is about keeping the practical benefits DPMA brings, without stopping production or raising costs through the roof. Anyone tired of headaches from paint fumes knows what’s at stake—so every step toward safer, cleaner solvents helps both workers and the planet.
Dipropylene Glycol Methyl Ether Acetate (often shortened to DPM Acetate) looks like a complicated name, but you’ll bump into it in everyday products. Factories use it to make paints flow smoother, inks dry slower, and cleaners more effective. This chemical drifts through plenty of workplaces; it sneaks into the air when coatings get sprayed or floors get scrubbed. Before letting worry take over, it’s worth looking into what this means for our lungs, skin, and general well-being.
It’s easy to shrug off a name like DPM Acetate if you’re not working with it every day. That said, I used to work in a print shop where the smell of fresh ink and solvents felt like wallpaper. We used gloves and tried to crank open windows whenever possible because too many folks complained about headaches after a shift. OSHA and the CDC both point out that breathing in a lot of these vapors, especially over time, isn’t a great life choice. Some people get dizzy or lose their appetite; some get itchy eyes and throats. If the contact goes on long enough, the body pushes back with stronger signals.
Direct skin contact rarely causes burns, but cracked and dry hands almost become part of the job for crews who skip gloves. If you splash DPM Acetate in your eyes, irritation follows almost right away. Swallowing this stuff by accident is the kind of scenario that lands someone in the emergency room. So yes, ignoring the hazards just isn’t smart.
There’s a clear difference between sniffing an open can once in a blue moon and soaking in this stuff daily. Animal studies show the liver processes DPM Acetate, but too much exposure harms kidneys and blood. No one wants to be a lab rat, even if humans can handle a little more of the vapor before real harm sets in. The safety bar sits lower for pregnant people; studies on similar chemicals link big doses to birth defects.
Some factory managers believe good air circulation is plenty, but masks and goggles still have their place. In a world scrambling for profit, workers sometimes trade their health for faster production. In my experience, the folks who don’t cut corners—who insist everyone wears gloves and checks the vent fans—have fewer sick days and headaches on the floor.
Every job site benefits from honest conversations about the dangers of chemicals like DPM Acetate. Clear labeling and updated training help everyone understand what they’re signing up for. Ventilation matters, but only as part of a bigger strategy that includes regular breaks, good hygiene, and the right gear. For anyone mixing their own paint at home, lots of fresh air and rubber gloves cut down risk in a big way.
It pays to push for safer substitutes, especially as new solvents come onto the market. Big companies hold the power to invest in research that gives workers better choices. Until all factory floors get updated, knowledge is a decent shield. I’ve watched workers grow more confident and less sick just by learning what’s in those bottles and how to handle them with respect.
Dipropylene Glycol Methyl Ether Acetate, often showing up in manufacturing plants or busy paint shops, demands respect. It’s easy to think you’ll be fine if you’re just working with it for a day, maybe even for an hour, but shortcuts around chemical safety have a way of catching up at the worst possible moment. In shops where this stuff flows from barrel to mixer, even the most seasoned worker keeps their guard up, and for good reason.
Nobody wants to head home with irritated skin or red, stinging eyes. Chemical-resistant gloves, a pair that doesn’t let liquids through, turns a risky job into a much safer one. There’s no need for fancy tech—rubber or nitrile gloves work. Eye protection earns its spot too, since just a splash can leave you blinking in pain or dealing with complications much later. Safety goggles with side shields cover what regular glasses can’t. From the first moment the drum cracks open, these ought to sit within reach.
Fresh air makes all the difference. Ventilation isn’t just about blowing dust around. Proper fans or exhausts pull fumes out, so nobody ends up with a headache or worse by lunchtime. For jobs in tight corners or any spot where fumes collect, a mask with organic vapor cartridges steps in as a shield for your lungs. Ignore this long enough and you’ll learn quickly how harsh some chemicals can feel in your chest.
Eating or drinking where chemicals are handled welcomes trouble. Dusts or small splashes jump off surfaces and can end up where they’re not wanted. There were days in my own shop when a forgotten sandwich picked up traces of solvent because someone left a lunch kit on a workstation. The best bet is to grab lunch away from the line, and give hands a good scrub—using soap and plenty of water—before digging in.
Open shoes or shorts have no place near strong solvents. I’ve watched folks regret that mistake more than once. Long sleeves, sturdy pants, and closed shoes keep most accidents minor, and prevent the surprise of a drop landing on bare skin. If clothing picks up the chemical, changing right away prevents slow burns or rashes from getting worse.
Drums and bottles should sit in cool, dry corners with clear labels. Spills become much harder to clean up when they seep out of damaged containers. I once saw a pallet soaked in solvent after someone ignored a cracked lid. That mess lasted for weeks, not just because of cleanup, but because the smell and the risk lingered on. Putting tools and containers back in the right spots cuts down on mix-ups and accidents, especially during busy shifts.
Spills still happen, even with the best routines. A kit with absorbent materials—think kitty litter or dedicated pads—handles small puddles fast. Once the mess gets cleaned up, sealing it in the right waste drum means it won’t end up somewhere it shouldn’t. Regular trash or a drain shouldn’t see anything that’s touched this chemical.
Nobody can memorize every hazard. A bit of steady training, refreshers each year, and honest talks about the risks lift everyone’s game. Folks who’ve seen accidents teach others why following those steps matters—not just to avoid penalties, but to make sure everyone clocks out healthy.
Many folks think handling solvents is only the job of scientists in lab coats, but everyday workers cross paths with them all the time. Dipropylene glycol methyl ether acetate, or DPM acetate for short, pops up a lot in coatings, paints, and inks. Every person who handles it—whether on a paint line, in a printing shop, or managing a warehouse—relies on proper storage to stay safe. Just doing what’s fastest or stacking drums in a dark corner doesn’t cut it. Small mistakes grow into big headaches, from equipment damage to health scares or even workplace shutdowns.
This solvent won’t burst into flames as easily as gasoline, but its vapors still catch fire if the setup is careless. Fumes can build up in closed-off spaces, especially if containers get left open or seals fatigue over time. I’ve seen spills around drum lids because someone stopped checking for leaks. The liquid itself can eat through some plastics, so trusting any old container can turn into a sticky mess by the next shift. You only need one pair of soaked work boots to realize why container choice matters.
Another sticky point comes from mixing. DPM acetate shouldn’t mingle with oxidizers and acids. Sometimes, it’s tempting to squeeze another barrel onto a shelf and hope for the best. Skipping the storage charts might look harmless at first, but those unseen chemical reactions don’t need help from anyone watching. From corrosion to dangerous fumes, the price for shortcuts always shows up later.
Most storage areas suffer from the same old problem—just enough air moving to hit minimum requirements. Some warehouses run hot in the summer or freeze over during winter nights. DPM acetate stays stable at room temperature, but extreme heat can change things. Vapors get restless and look for ways out. Without enough airflow, every small leak becomes a problem.
Real safety comes from regular walkthroughs. Someone should check that vents on drums work and no mystery puddles creep up. It’s tempting to leave ventilation fans off to save electricity, but stale air turns simple mistakes into big problems, especially if the warehouse backs up to the production floor.
Correct labeling sounds obvious, but after a busy shipping day, drums without fresh stickers get lost in the shuffle. Workers new to the job may not know the difference between one solvent and another unless every container stands out. Training helps, but so does clear signage. Everyone on the floor should know which solvents demand gloves, which ones sting eyes, and which ones start fires.
Spill kits hide under benches too often. Employees need to know what’s inside and actually feel comfortable grabbing them. No rulebook keeps anyone safe if folks have to search for instructions during an accident.
We can talk about policy all day, but change happens in daily decisions. Investing in dedicated chemical storage cabinets with real ventilation, not just cracked windows, makes sense. Tracking drum movement and practicing spill response helps everyone know their roles. Above all, no one should face hazardous vapors or accidental fires because someone thought a shortcut sounded good. Experience teaches the hard lessons; safer storage lets people dodge that pain before it hits home.
Anyone with a background in paints, inks, or coatings likely bumped into dipropylene glycol methyl ether acetate at some point. Around shops and workshops, folks call it DPMA or DPM Acetate for short. Chemists like it for its low odor, moderate evaporation rate, and high solvency. Anyone curious about its compatibility with other chemicals doesn’t need to dig too far to see trouble or harmony cropping up.
Mixing any solvent with other chemicals gets tricky if you don’t know what you’re doing. DPMA seems pretty friendly — it mixes well with a lot of common organic liquids, things like esters, ketones, alcohols, and glycols. In the day-to-day, formulators often pair DPMA with ethyl acetate or butyl acetate, and don’t bat an eyelash about gumming up the system.
Give DPMA some water, though, and it reveals a more temperamental side. Plenty of glycol ethers, including DPMA, show only limited water solubility. Leave the mixing to chance and you might end up with cloudiness or phase separation, especially in cooler spots of the plant or lab. This has tripped up more than one rookie technician, especially during winter.
On the surface, DPMA looks mild-mannered. Throw acids or strong bases into the mix, and you can end up with hydrolysis or unwanted reactions that cut down solvent power. I’ve seen tanks of in-progress coatings ruined by a slip-up on pH control. DPMA doesn’t care for strong oxidizing agents either—peroxides, chlorine, and the like will chew right through it, turning a useful tool into a problem.
What about plastics? DPMA attacks certain types, especially lower-grade PVC or some rubbers. Folks handling plastic lines or seals stumble onto leaks or swelling after a few weeks. I’ve seen pump fittings fail early because someone forgot to check chemical resistance charts.
Every time a new recipe gets built, it pays to keep a compatibility chart nearby. These days, many suppliers hand out one-pagers with recommendations spelled out. DPMA often earns good marks as a compatibilizer — it helps dissolve pigments or resins by bridging differences between other solvents. In flexible packaging inks, for example, DPMA teams up with fast-evaporating acetates to make a more workable blend.
Once, working with a client formulating wood stains, we tried swapping the DPMA for a cheaper glycol ether and the pigment flocculated the moment the resin dropped in. Some lessons cost money. Compatibility doesn’t just come down to chemistry charts, either — temperature, humidity, and order of addition all stir the pot in real-life plants.
When something goes wrong, the root cause nearly always circles back to skipping a basic test: mixing a small sample and waiting overnight to spot changes. Pouring a batch together just because two chemicals look similar on paper can burn an entire day’s production. Engineers in paint plants now keep samples in glass jars, checking not just for clarity or viscosity shifts, but also for separation, odor, and film-forming ability.
So next time someone wonders about DPMA’s compatibility chops, a bit of old-fashioned lab work goes a lot further than guesswork. Start small, blend carefully, and if you spot any trouble — stop before dumping in a drum’s worth.
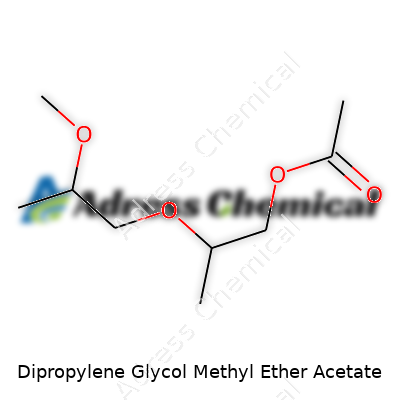
| Names | |
| Preferred IUPAC name | 1-(2-Methoxy-1-methylethoxy)propan-2-yl acetate |
| Other names |
DPM Acetate Dipropylene Glycol Monomethyl Ether Acetate Propylene Glycol Methyl Ether Acetate 1-(2-Methoxy-1-methylethoxy)propan-2-yl acetate Dipropylene glycol methyl acetate |
| Pronunciation | /daɪˌproʊpiːliːn ˈɡlaɪˌkɒl ˈmɛθəl ˈɛθər əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 88917-22-0 |
| Beilstein Reference | 1811220 |
| ChEBI | CHEBI:81313 |
| ChEMBL | CHEMBL185972 |
| ChemSpider | 5464046 |
| DrugBank | DB14006 |
| ECHA InfoCard | 13faac5c-53aa-4a85-b16a-f9e8a53f47c5 |
| EC Number | # 89637-37-2 |
| Gmelin Reference | 105995 |
| KEGG | C18607 |
| MeSH | D004083 |
| PubChem CID | 8294 |
| RTECS number | KN2250000 |
| UNII | 6RZ6XEZ3CR |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C10H20O4 |
| Molar mass | Molar mass: 204.26 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Faint ester-like odor |
| Density | 0.951 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.36 |
| Vapor pressure | 0.03 mmHg @ 20°C |
| Acidity (pKa) | pKa ≈ 16 (estimated, very weakly acidic) |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -7.58×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.406 |
| Viscosity | 0.95 mPa·s (at 25°C) |
| Dipole moment | 2.92 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -745.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4549.7 kJ/mol |
| Pharmacology | |
| ATC code | D01AE15 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H336 |
| Precautionary statements | P210, P261, P271, P280, P301+P312, P304+P340, P312, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 81°C |
| Autoignition temperature | 179°C |
| Lethal dose or concentration | LD50 (oral, rat): 5,192 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5,190 mg/kg |
| NIOSH | NIOSH: TX 4022500 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Dipropylene Glycol Methyl Ether Acetate: 100 ppm (TWA) |
| REL (Recommended) | 25 ppm |
| Related compounds | |
| Related compounds |
Dipropylene Glycol Methyl Ether Propylene Glycol Methyl Ether Acetate Ethylene Glycol Methyl Ether Acetate Tripropylene Glycol Methyl Ether Acetate Diethylene Glycol Methyl Ether Acetate |