Chemists first set eyes on Dipropylene Glycol Dimethyl Ether back in the mid-20th century, at a time when the search for safer and more versatile solvents was running hot. Production increased as leaders in pharmaceuticals and electronics saw its value, especially after environmental pressure around hazardous solvents picked up steam. Among seasoned chemists, stories circulate about late-night experiments with new ethers—some with dangerous volatility, others too stubborn to dissolve anything useful. This one, sometimes called DMM or DPGDME, managed to strike a careful balance. Its stability, combined with decent solvating strength, convinced many researchers to swap out old, nasty chemicals in favor of something less threatening. Over the years, as green chemistry gained ground, industry insiders turned to Dipropylene Glycol Dimethyl Ether to comply with stricter rules on emissions and workplace safety.
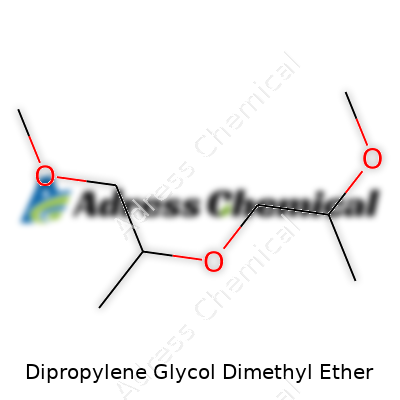
In laboratory cabinets and factory drums, Dipropylene Glycol Dimethyl Ether, known by CAS number 111109-77-4, stands out as a colorless liquid with a mild, almost elusive odor. Chemists pick it up for its reputation as a safe, easily handled alternative to more extreme solvents. The molecule contains two propylene glycol units joined with an ether link, each capped by a methyl group. This little piece of organic engineering delivers remarkable compatibility with resins, polymers, and a broad selection of organic molecules that need dissolving or dispersion. In real-world terms, it turns sticky, tough-to-handle ingredients into manageable, pourable liquids. Its popularity in the lab owes much to its broad utility—it helps resolve the headache of finding one solvent to handle both polar and non-polar compounds.
Pour a sample of Dipropylene Glycol Dimethyl Ether and you notice an almost water-clear substance, with a low viscosity that pours easily even in cold rooms. Its boiling point hovers around 175°C, high enough that it works in heated processes but low enough for distillation if recovery is needed. Water won’t pull it away—it exhibits low miscibility with water, yet handles most organic compounds like a pro. Chemically, it stands firm in the face of weak acids and bases, shrugging off reactions that other ethers can’t survive. Yet, it never gets too cocky—under strong acids or with powerful oxidizers, it breaks down, as do most ethers. Its vapor pressure and flash point meet requirements for industrial safety, making large-scale use practical for most facilities.
Every drum, flask, or IBC container of Dipropylene Glycol Dimethyl Ether should wear a detailed label. Suppliers mark purity percentages, typically exceeding 99% for industrial-grade material. Other standard metrics like water content (often less than 0.1%), color index, acidity, and distillation range face close scrutiny from quality assurance staff. Packaging comes in steel or HDPE drums with hazard labels indicating its flammable status, and safety data sheets spell out storage, handling, and spill response steps. Companies seeking regulatory approval in countries like the US, EU, or China face slightly different compliance tests, though the core properties stay the same across borders.
Dipropylene Glycol Dimethyl Ether isn’t found lying around in nature, so chemists start with a building block like propylene oxide. They react propylene oxide with methanol through a carefully controlled catalytic etherification. Watching a batch process run, engineers monitor pressures, temperatures, and flow rates so byproducts don’t sneak through. After the reaction, distillation columns help purify the target molecule, separating it from excess methanol and other side products. Workers in these facilities learn to trust the control room’s readings: slight temperature drift or off-spec feedstock can throw off yields and squeeze profit margins fast.
The chemical backbone of Dipropylene Glycol Dimethyl Ether behaves as a reliable, stable ether for most reactions, not prone to sudden spikes in reactivity. In synthetic chemistry, people rely on this quality when carrying out processes like Grignard reactions, which need inert surroundings. Attempts to tweak the molecule usually target the methyl groups or the ether link, although this isn’t standard practice in larger industry. When required, strong acids or oxidizing agents break the molecule into smaller fragments—a consideration for waste treatment. Over-enthusiastic heating poses a risk of forming peroxides, so many labs make it routine to test older stocks, protecting both people and experiments.
Industry holds onto a handy list of names for Dipropylene Glycol Dimethyl Ether. Aside from its IUPAC mouthful and abbreviation (DPGDME), trade catalogs mention terms like Di(propylene glycol) methyl ether or 1-Methoxy-2-(2-methoxypropoxy)propane. Some suppliers lean into their own branding—“Dowanol DPM” or “Arcosolv DPM” show up on drum labels. Chemists learn to recognize the common codes and product sheets even faster than they pick up on chemical formulae, simply because mistakes in ordering mean missed deadlines and lost money.
Everyone dealing with Dipropylene Glycol Dimethyl Ether needs to show the same respect given to any flammable liquid. Eye protection, gloves, and fume hoods keep accidents at bay. Regulations demand storage away from open flames and oxidizers, and solvent waste needs labeling so it doesn’t get mixed up in general trash. Training sessions hammer home the risks of vapor exposure and skin contact, though years of safe handling show it avoids the health nightmares of some solvents used decades earlier. Workers coming home from a day in production rarely complain of chemical odors lingering in their hair or skin, which speaks volumes compared to some of the harsh compounds it replaced.
Product developers across fields from coatings to electronics rely on Dipropylene Glycol Dimethyl Ether for its ability to dissolve resins, aid in ink formulations, and keep high-performance adhesives stable through changing seasons. In lithium-ion battery manufacturing, this solvent speeds up the mixing process for electrolytes, critical for smooth cell assembly. In cleaning formulations, it proves worth its weight for removing greasy, stubborn residues, outperforming traditional alcohols in both industry and household cleaning wipes. Paint technologists use it to fine-tune drying times and surface gloss—something only the most experienced can balance by intuition. In pharmaceutical research, workers value its chemical mildness, making it a go-to carrier for sensitive intermediates.
Scientists haven’t stopped looking for even better uses for Dipropylene Glycol Dimethyl Ether. Many labs focus on making greener solvents, and this one stands out for its low toxicity and smaller environmental impact when compared to classic ethers or hydrocarbons. Modern research investigates its performance in lithium-ion batteries, polymers, and pharmaceuticals. Teams publish data showing tweaks to formulations that boost energy efficiency in batteries or improve coatings for electronics. Some environmental chemists run lifecycle analyses, aiming to close in on solvents that align with circular economy goals. In large company R&D centers, trial runs of greener product lines often feature DPGDME, especially as regulations around emissions get tougher every year.
Lab data and real-world case studies both suggest Dipropylene Glycol Dimethyl Ether poses a much lower risk to health than old-line solvents, such as benzene or toluene. After years of tests with rodents and fish, regulatory bodies classify the material as having low acute toxicity. Occupational safety data highlight mild skin and eye irritation with high or prolonged exposure, though accidental spills don’t tend to trigger medical emergencies. Disposal regulations still call for careful handling, simply because the legacy of industrial solvents includes environmental contamination that lasts generations. Toxicologists keep tabs on long-term, low-dose exposures, especially as usage ramps up in green chemistry and electronics plants. There’s always a push for more data, and as volumes increase, risk assessments adapt to new realities.
Demand for greener, performance-driven solvents grows by the month. Experience tells me that suppliers and manufacturers gravitate towards substances like Dipropylene Glycol Dimethyl Ether, where safety and performance aren’t locked in a battle. Regulation continues tightening, and companies know that swapping in safer chemicals now avoids government headaches and expensive recalls down the road. R&D budgets for new battery chemistries, coatings, and low-VOC products all include funds for solvent selection—a trend I’ve watched unfold more rapidly in the last decade. Forward-looking producers set aside capacity for DPGDME, betting not only on trends but also on sheer practicality. A generation of chemists raised on green chemistry expects tools that won’t sacrifice convenience or push new regulatory risk onto future workers or communities. In the next wave of product launches and material breakthroughs, this versatile ether looks set to keep playing a central role wherever clean, reliable solvent action is required.
People tend to overlook the role of chemicals that help things blend or clean up quickly. Dipropylene glycol dimethyl ether—sometimes labeled as DPGDME—doesn’t attract attention like flashy new tech or viral trends. But take a peek behind the curtain of industries like electronics, paints, and cleaning products, and you’ll find it getting plenty of work done.
In circuit board production, workers use DPGDME as a solvent to rinse off flux residues and keep connections reliable. You won’t see circuit makers bragging about their solvents, but without the ability to remove grime and soldering debris, you end up with unreliable gadgets or things that fall apart faster than they should. The industry looks for solvents that are less aggressive but still able to clear tough residues, which helps prevent damage to sensitive micro-components. DPGDME comes in because it doesn’t eat away at delicate surfaces, which is a common complaint with harsher cleaners. Less damage means less waste and better product lifespan.
If you’ve ever painted a fence or refinished a table, a smooth, even finish is usually the goal. DPGDME ends up in plenty of paints and varnishes because it helps dissolve pigments and binders, so you get fewer lumps and streaks. It evaporates slower than standard solvents, extending drying time. In hot climates, this extra time means paint doesn’t dry and crack before you’re done. The result? Fewer do-overs and less frustration on summer projects.
Some household degreasers or glass cleaners swap out harsher chemicals for DPGDME to lower strong smells. Nobody wants their kitchen reeking of chemicals after cleaning the counters. DPGDME’s low odor and solid ability to dissolve oily messes make it a good fit for these products. It helps bust up grease and residue without turning daily chores into a nose-pinching event.
It’s not all sunshine with any industrial solvent. DPGDME may be less flammable and less volatile, but workers still need gloves and good ventilation when handling it. Those with sensitive skin, like me, should pay attention—one accidental splash, and the irritation can last for hours. Anyone working around solvents long-term needs extra health protections and solid training. This is not just bureaucratic red tape—it’s about keeping people healthy in the long run. I’ve seen coworkers cut corners on PPE and end up regretting it for days.
As regulatory pressure mounts and more folks pay attention to chemical safety, companies are on the hunt for alternatives—either solvents from renewable sources or formulas that biodegrade faster after use. A transition like this won’t happen overnight. Labs test substitutes for years to make sure they work just as well and don’t trigger new health concerns. I’ve talked to researchers who say some “safer” solvents fall flat when put to the test, clogging up machinery or leaving behind streaks. The search for the right combination of performance and safety keeps DPGDME and its relatives around, at least for now.
Chemicals like DPGDME keep modern life humming, even if they don’t show up in glossy ads. Behind electronics, paints, and cleaners, workers, manufacturers, and safety advocates face real choices about what goes into the products that land on store shelves. The more we pay attention to the ingredients on labels or safety sheets, the better chance we have at driving change for the safer, smarter solutions yet to come.
Anyone who’s spent time in a workshop or lab gets to know the story chemicals carry. Dipropylene Glycol Dimethyl Ether, or DPGDE for short, shows up in places you’d expect: coating factories, battery research benches, ink mixing rooms. Its job: break things down or help other chemicals blend. It evaporates slowly and barely smells, which might lull some into treating it like water. The truth goes deeper.
DPGDE is not something you want sitting on your skin. Even if it slips under regulatory radars more than old-school solvents like toluene or benzene, people can run into trouble. There are reports of skin dryness, irritation. Protective gloves aren’t something I’ve just read about—I’ve had to use them myself, scrubbing sticky hands after spills that left skin raw. On a direct exposure level, it’s not the nastiest thing out there, but nobody comes out better ignoring its risks.
Eyes feel the sting too. Vapors might sneak under the radar, especially in stuffy indoor setups. Anyone with asthma in the family gets wary of new solvents, and this one is no different. In small spaces, prolonged use triggers complaints, headaches, and irritation. I remember visits to print shops where small exhaust fans struggled to keep air clear—ventilation never feels overdone till you don’t have it.
The data is out there: DPGDE scores low toxicity on some charts, but most long-term health studies admit they don’t know everything yet. Animals show symptoms at high doses, especially with repeated contact. Most safety sheets urge caution—goggles, gloves, long sleeves, proper disposal, and, most vital: don't breathe the vapor. The solvent’s ability to dissolve grease and ink also means it slips through ordinary latex gloves, carrying other nasty substances with it.
Fire risk is real. DPGDE doesn’t catch fire as quickly as acetone, but given a hot surface and vapor buildup, it will burn. Seen enough charred benches and singed eyebrows to treat every solvent as a spark away from trouble. Forgetting to check the flash point or storing open drums near a furnace turns a shortcut into a disaster.
Old habits can backfire. Pouring DPGDE without a fume hood or skipping eye protection can teach lessons nobody asks for. Workers using it day in, day out, get complacent. Shops do well by investing in good gloves—lined nitrile holds up better than basic options. Decent goggles don't cost much. I always kept a big bottle of hand cleaner nearby; cheap insurance for fast, thorough cleanup.
Training makes all the difference. Companies offer the bare minimum—one-page data sheets, a warning sticker, maybe, if you’re lucky. Trouble starts when people only skim the rules. I’ve always found it’s best to talk through the process, point to where the ventilation kicks in, remind each other that “I’ll be quick” never made anyone safer.
Waste disposal deserves attention. Dumping old DPGDE down the drain is the fast route to big fines and groundwater headaches. Most cities have hazardous waste drop-offs for good reason. Proper sealed containers save a lot of legal worry, and keep the neighborhood safer too.
Solvents like DPGDE earn their spot for a reason. With the right gear, clear routines, and healthy respect for what’s in the drum, DPGDE can be used without drama. The trap lies in underestimating something because it lacks a strong smell or immediate bite. Nobody remembers personal safety gear until they forget it once—then the lesson lingers. A safe space for chemicals comes down to habits, not luck or ignorance.
Dipropylene Glycol Dimethyl Ether—or DPGDME, if you want to save a few syllables—often catches attention in labs and manufacturing rooms. You won’t see it on grocery store shelves, but it powers a surprising number of modern processes. Clear as water, practically odorless, and with an unassuming look, this liquid backs up its plain style with a long list of talents. I remember the first time I handled DPGDME: gloves on, fume hood running, and everything feeling just a bit smoother. That's because DPGDME goes easy on your equipment, isn’t too picky about who it mixes with, and won’t leave behind sticky messes.
The liquid's colorless nature actually speaks to its purity. With a boiling point hanging around 180°C, it keeps its cool in a hot environment. I’ve seen it warm up nicely during extended reactions, but it sticks around longer than you’d expect from an ether. Its flash point sits above many other ethers, at roughly 75°C, which means there’s a bit more breathing room before flammable vapors become worry-worthy. Handling solvents like DPGDME in the lab or at a plant comes with fewer jumpy moments because they don’t light up the second you turn your back.
Its viscosity feels similar to water or a gentle syrup. Pouring it into a beaker, it won't rush out as quickly as pure water, but still slips around far more easily than thick glycols. Its density rests around 0.94 g/cm³. This means it floats close to water but not quite on top, making mixing and phase separation more manageable when the job calls for it.
Solubility marks another perk. DPGDME blends well with many organic solvents—things like alcohols, esters, and ketones get along well with it. You also get mild solubility in water itself. That little trick comes in handy for cleanup and for reactions where a touch of water might sneak in. Some solvents stubbornly refuse to mix, but DPGDME isn’t one of them; it stands out as a real team player in multi-phase chemistry setups.
Chemically, DPGDME doesn’t attract much drama. Unlike simple ethers, which sometimes turn into peroxides if you let them wait too long, DPGDME shrugs off most attempts at destabilization. I remember keeping a bottle tucked in the back of a solvent cabinet for months, and it went right back to work without any fuss or mystery crystals forming on the cap. That reliability makes life easier for chemists dealing with air-sensitive or reactive compounds. Its stability also means DPGDME remains valuable for storing sensitive reagents or performing tough reactions, especially with strong bases or acids that might wreck other choices.
From an environmental and safety perspective, this solvent edges ahead of many old-school chemicals. While DPGDME burns, the risk sits lower than with diethyl ether or similarly volatile liquids. It also tends to break down more gently in nature, without leaving behind persistent toxins. Occupational safety sheets still recommend ventilation, gloves, and goggles, but the risk of headaches, chronic irritation, or fires drops down a few notches compared to more hazardous cousins.
Despite sporting an attractive profile, DPGDME brings challenges that shouldn’t be glossed over. Disposal requires care, since dumping solvents can still leach chemicals into groundwater or trigger regulatory headaches. Better recycling programs, coupled with investment in alternatives, could keep solvent use more environmentally friendly. Companies ought to keep an eye on improving distillation recovery technologies, cutting down on the volume of spent solvent that gets thrown out. For labs, education about greener choices and safer handling routines helps protect both workers and the environment. There’s plenty of promise here, and with just a bit of clever work, DPGDME can continue to be a safer, more effective tool for years to come.
Dipropylene Glycol Dimethyl Ether, often seen as DMM or DPGDME, isn’t your everyday household chemical. This solvent shows up in labs, manufacturing plants, and tech companies. People often focus on how it helps certain reactions or dissolves tough-to-mix ingredients. The bigger conversation circles around safety, because this liquid brings flammable vapors and possible health risks along for the ride.
Take a tour through any well-organized workshop or chemical storeroom, and the first thing you’ll spot is some level of planning. Folks who’ve stored DPGDME for the long haul know that things go wrong fast without clear rules. A friend once told me about a warehouse fire kicked off by poor labeling and a forgotten leaky cap. Nobody wants to deal with insurance nightmares and government fines, let alone hospital visits. Avoiding that all starts with good storage.
Plastic softens and reacts with strong solvents over time. Thin metals rust or corrode when exposed to fumes. What works? Tight-sealed, unblemished containers built out of stainless steel, glass, or heavy-duty plastics. No one wants mystery ooze on the shelf or fumes leaking out and hanging in the air. I’ve learned, after spilling my fair share of chemicals, that easy-turn caps don’t cut it. Gaskets, heavy thread, and a quick label let everyone know what’s inside.
Heat fires up trouble. Warm rooms or spots near hot equipment turn liquid like DPGDME into a flammable vapor cloud. I saw one toolbox on top of a solvent drum once—just inches away from an overheating furnace. Stack solvents far from heat, and check thermometers often. Dry storage pulls double duty: water could lead to weird reactions or let corrosion attack the container. So, a cool, shaded corner with low humidity wins every time.
Great seal on the container solves half the risk. The other half depends on the room it sits in. I spent a summer in a lab where no one could crack open a window. Everyone’s eyes stung by noon thanks to the fumes. Air must travel—fans, vent hoods, or at the very least a good cross-breeze. Some companies run sensors that track air quality. Even a plain exhaust fan can go a long way toward keeping people safe.
Crowded shelves encourage mix-ups—wrong bottles reaching the wrong hands. Strong oxidizers, acids, or fuels sitting next to DPGDME? A recipe for trouble. One misstep could mix chemicals and spark a fire or create toxic gas. Color-coded bins or shelves, spaced well apart, stop the morning rush from becoming an emergency.
No label, no luck. Quick action in an accident rests on knowing what’s what. Clear labels—big, waterproof, and always facing out—tell anyone walking by what risk they're facing. Right near the storage spot, emergency kits with spill pads, gloves, goggles, and a fire extinguisher cut down on panic if something leaks or tips.
Regulations don’t spring up out of nowhere. They react to real accidents and close calls. Good storage practices for DPGDME aren’t just box-checking exercises. They keep workplaces running, limit wasted money, and protect people who’d rather avoid the ER. Whether working in a high-tech plant or a small garage, careful storage turns one more invisible danger into something manageable.
Dipropylene Glycol Dimethyl Ether, a bit of a mouthful for sure, actually pops up in more places than most people realize. Walk into any body shop or construction site and you’ll notice the importance of paint and coatings. High-performance paints don’t just stick on their own; solvents carry those pigments and resin onto surfaces, help level things out, and make sure things dry without ugly bubbles. Companies making industrial paints turn to this chemical for its ability to dissolve both water-based and oil-based materials. It keeps paint stable in the can and helps artists—whether they build bridges or refinish cabinets—avoid wasted time dealing with clumpy or uneven results.
Few people think about solvents when scrolling on a phone or working on a laptop. Still, the electronics industry leans hard on chemicals that clean and prepare circuit boards. Manufacturers use Dipropylene Glycol Dimethyl Ether to strip away greasy fingerprints, remove soldering residues, and dry parts without streaks. It evaporates at the right pace and leaves little behind. Electric vehicle batteries and solar panels, two of the world’s fastest-growing technologies, also benefit from its ability to dissolve specialty polymers and electrolytes, so engineers trust it in situations where purity is non-negotiable.
Making medicine takes more than recipes and laboratories. Synthesis steps rely on solvents to carry out reactions, separate compounds, and isolate the pure stuff. Modern drug companies try to avoid nasty, hazardous chemicals at all costs. This ether catches attention thanks to its low toxicity and its ability to handle both water- and oil-soluble ingredients. Mixing and separating chemical ingredients gets trickier each year as regulations tighten. Using this chemical, labs cut down on environmental risk but still get the precision results that medicine demands.
Hospitals, restaurants, and factories need to stay spotless to keep people healthy and machines running. Harsh degreasers break down toxic residues but often damage surfaces or pollute air. Here, Dipropylene Glycol Dimethyl Ether shines. It strips away baked-on food and oil, cleans delicate lab glassware, and even helps auto mechanics tidy up engine parts without breathing in harsh fumes. Companies look for products that work fast and rinse away clean, all without leaving behind noxious odors that drive workers out of a room.
Anyone paying attention to health and safety recalls knows that industry standards get tighter every year. More consumer goods companies swap out legacy chemicals for options that pass stricter tests. That means rethinking solvent use in everything from printer ink at the office to adhesives in flooring. Many manufacturers started shifting to Dipropylene Glycol Dimethyl Ether because it delivers the performance they remember but with easier handling and fewer headaches over disposal or storage.
Balancing performance, safety, and cost keeps companies in a constant state of adaptation. Governments encourage this switch by updating lists of approved solvents and offering tax breaks for “greener” options. I remember working alongside teams worried about shifting processes, but gradually, as replacement solvents like this one proved their value, confidence grew. Problems like supply chain snags and training still pop up, but improvements in workplace safety and environmental impact give workers and customers real reasons to support the change. Every bottle of paint, bottle of pills, or window-cleaning wipe that gets safer means one less worry for families and businesses alike.
| Names | |
| Preferred IUPAC name | 1-methoxy-2-(2-methoxypropoxy)propane |
| Other names |
DPGDME Proglyme Di(propyleneglycol) dimethyl ether 1-Methoxy-2-(2-methoxypropoxy)propane |
| Pronunciation | /daɪˈproʊ.piːliːn ˈɡlaɪ.kɒl daɪˈmɛθ.əl ˈiː.θər/ |
| Identifiers | |
| CAS Number | 111109-77-4 |
| Beilstein Reference | 1208737 |
| ChEBI | CHEBI:83883 |
| ChEMBL | CHEMBL168790 |
| ChemSpider | 123591 |
| DrugBank | DB14135 |
| ECHA InfoCard | 100.104.226 |
| EC Number | Energy Drinks |
| Gmelin Reference | 83184 |
| KEGG | C19613 |
| MeSH | D003976 |
| PubChem CID | 8215 |
| RTECS number | JM9235000 |
| UNII | X60I3P2CQV |
| UN number | UN2325 |
| CompTox Dashboard (EPA) | DTXSID7046660 |
| Properties | |
| Chemical formula | C8H18O3 |
| Molar mass | 162.23 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.951 g/cm³ |
| Solubility in water | Miscible |
| log P | 0.6 |
| Vapor pressure | 0.42 mmHg @ 25°C |
| Acidity (pKa) | Acidity (pKa): 17.1 |
| Basicity (pKb) | pKb: 2.90 |
| Magnetic susceptibility (χ) | -7.84 × 10⁻⁶ |
| Refractive index (nD) | 1.400 |
| Viscosity | 1.5 mPa·s (25 °C) |
| Dipole moment | 4.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -460.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4775.6 kJ/mol |
| Pharmacology | |
| ATC code | D08AX99 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 77°C |
| Autoignition temperature | 160 °C (320 °F) |
| Explosive limits | 1.3% - 10.1% |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 5,400 mg/kg (oral, rat) |
| NIOSH | UNII9S1M1W7KC9 |
| REL (Recommended) | '≤ 200 mg/m³' |
| Related compounds | |
| Related compounds |
Diethylene glycol dimethyl ether Dimethoxyethane Dioxane Tetrahydrofuran |