Folks in the chemical world noticed the need for flexible, low-toxicity plasticizers almost a century ago. The story of diisobutyl adipate goes way back to a time when industries raced to produce safer, more versatile esters for plastics, cosmetics, and even pharmaceuticals. It didn’t take long for manufacturers to favor this particular adipate over high-volatility compounds that were giving headaches in both literal and regulatory senses. During those decades, manufacturers relied on trial and error, shifting methods and feedstocks.
By the time synthetic polymers found themselves at the core of many products, diisobutyl adipate stepped in as a smart compromise. Oil-based phthalates earned a bad reputation, especially as health studies started piling up. Consumer pushback sent engineers, chemists, and entrepreneurs searching for alternatives that wouldn’t strip away the performance or workability needed for coatings, lubricants, or personal care goods. Diisobutyl adipate proved to be a smart move, allowing for easier processing in hot and cold climates. Every big chemical shift started with incremental, often stubborn, experimentation, and this compound survived that tough vetting process.
Diisobutyl adipate lives as a flexible, colorless liquid. It's part of a family called adipate esters, acting as a plasticizer—basically, it gives rigidity a break and lets products like films, lotions, and even inks move, stretch, and apply without flaking or cracking. It’s sought in formulations for skin care, polymers, and adhesives. Plenty of formulators pick it because the stuff stays light, keeps a pleasant skin-feel, and doesn’t come bundled with strong, lingering odors. It finds its way in finishings for shoes, toys, hydraulic fluids, and more.
Diisobutyl adipate brings a low viscosity and a very subtle scent that doesn’t overpower finished products. The compound’s boiling point sits around 320°C. Its melting point floats below -60°C, making it valuable for anything you need to use in cold temperatures. Miscibility matters for every chemist and blender, and here you get decent solubility in most common organic solvents. The density run at room temperature hovers close to 0.98 g/cm³. Its refractive index rounds out the picture in the normal range for esters. For those keeping an eye on flammability, flashpoint sits a bit above 170°C. These aren’t just trivia—they determine where and how this material actually works.
Stability comes easy for diisobutyl adipate under most typical storage situations. It resists hydrolysis far better than other adipates, so you don’t get degradation that leads to sticky messes or sticky regulatory situations. Chemically, the ester linkage stays robust unless you let acids or bases get out of hand in the workplace.
Buyers in the supply chain ask for a clear list: purity usually above 98%, minimal residue after evaporation, and absence of heavy metals. You’ll see these figures splashed across product datasheets—each percentage point means something to someone down the line, whether that’s for a sunscreen, a PVC gasket, or a batch of hot-melt adhesive. Most packaging carries standard Global Harmonized System (GHS) labeling. Hazard pictograms tell you about flammability and possible mild irritation risks. Some vendors throw in QR codes and batch histories, which isn’t extra—it’s practical. This transparency means workers can quickly trace any hiccup back to the original barrel or lot. European and U.S. safety data sheets read almost the same, save for some language around exposure limits, though both explain spill cleanup and ventilation needs.
Creating diisobutyl adipate usually means starting with adipic acid and topping it off with isobutanol, cooked up in the presence of a catalyst. Plenty of factories run these syntheses in reactors fitted for careful temperature control. Sulfuric acid or p-toluenesulfonic acid push the reaction ahead, driving water out and keeping the yield decent. Some processes recycle excess alcohol, using distillation to capture and reintroduce the input. The reaction releases water, so removing that keeps the conversion clean. Each run gets checked for unwanted byproducts—if acids or unconverted alcohols linger, it means a rerun or extra purification.
The purification usually runs through stripping and filtration. Any color or odor left in the finished product lands it in the “rework” pile. If you’re in a region with stricter environmental rules, you’ll see investments in scrubbers or water treatment to capture waste. Not glamorous work, but it means no discharge causes downstream headaches.
Chemists often tinker with adipate esters, and diisobutyl adipate is no exception. Its backbone opens up a few possibilities—some tweak it further for specialized polymer chains or custom lubricant blends. The ester linkage stands up to mild conditions, but if strong acids or bases show up, hydrolysis breaks it back into adipic acid and isobutanol. Engineers interested in extending working ranges for gear or engine fluids sometimes modify the molecule to adjust solubility or volatility, working toward better resistance to environmental stressors. Some research labs use the parent compound for further esterification or transesterification, getting newer additives for paint or ink formulations.
Others throw it into copolymer blends, letting bulk plastics gain elasticity or smoother application. These downstream changes give manufacturers a broader toolkit—light new esters for greener lubricants, softer polymers for safer kids' products, or resilient coatings that last longer under sun and sweat.
Manufacturers and buyers might call it by several other names on paperwork: hexanedioic acid, diisobutyl ester, DIBA, or just adipic acid diisobutyl ester. A few brand names pop up in catalogs, mostly for use in skin care or industrial blends. Each label usually points to the same chemical, but formulators check batch specs to avoid any surprises.
Safety means practical steps: keeping proper ventilation, checking gloves, and avoiding splashes in the eyes. Exposure data shows low acute toxicity, though it can cause minor skin or eye irritation for some people. Companies train workers to handle spills with absorbent material and to avoid letting the material seep into drains. Regulations in Europe, the U.S., and much of Asia ask for regular airborne concentration checks—usually using closed system transfers and smart exhausts. Long exposures in confined spaces could cause headaches, but those usually come from vapor concentration levels nobody should allow in a well-run facility.
Storage guidelines turn simple: keep containers sealed, cool, and away from open flames. Labels show required pictograms and long chemical names, but in real life, it’s about making sure nobody stacks barrels too high or lets a leaky drum slip through. Newer safety practices add QR tracking, integrating supply chain transparency with actual on-site safety audits. These digital additions move beyond paperwork, enabling quicker recalls and targeted warnings if anything turns up off spec.
Polymer manufacturers use diisobutyl adipate to keep PVC pliable in cable coatings and vinyl toys. Cosmetic chemists call on it for sunscreen, body lotion, and roll-on formulations—it leaves a silky finish and doesn’t fade fragrance profiles. In the coatings industry, it acts as a low-viscosity carrier, enhancing spreadability without slowing down curing or leading to surface imperfections. In the lubricant sector, it works as a low-temperature additive for specialty greases needing flexibility under chillier than usual conditions. Adhesive makers tap its properties to balance peel strength and stickiness for film applications.
Pharmaceutical companies select it for some topical and transdermal products due to its skin-compatibility. Food-contact films and wraps have strict standards, but adipates get a second look when legacy plasticizers face tighter regulations or outright bans. This versatility keeps demand solid, as sectors pivot away from phthalate-based ingredients and look for options with established safety records.
Recent years brought a rush of innovation as industries looked for blends that work across climate zones and meet ever-changing eco-standards. Materials labs test diisobutyl adipate in complex formulations, pushing its limits under stress, UV, and at low temperature extremes. Formulators and regulatory experts look closely at each degradation product—if a new impurity pops up, they run tests to check for allergenicity or long-term effects. Startups try bio-based adipic acid and isobutanol to make production cleaner, aiming for lower carbon footprints. This development isn’t happening in isolation—companies bring in green chemistry principles, life-cycle assessments, and even blockchain record-keeping to prove performance and sustainability to tough modern buyers.
Several research groups examine how blends using diisobutyl adipate fare in next-generation coatings for renewable energy equipment. Others are running comparative trials on new polymer films, checking migration rates, and how the material behaves in contact with foodstuffs or pharmaceuticals. Some look at deeper use in biodegradable packaging, pairing the old with the new.
Animal and lab studies so far paint a reassuring picture of diisobutyl adipate’s safety. Acute toxicity sits low, with only mild, temporary effects observed in inhalation or skin contact exposures at concentrations higher than typical occupational settings. Chronic exposure studies dig into long-term effects, where researchers track hormone activity, metabolic pathways, and overall carcinogenic potential. Current findings put it solidly in the “low-concern” group compared to legacy plasticizers, especially the much-maligned phthalates. Government reports from the European Chemicals Agency and the U.S. EPA both support these assessments.
Critical voices always urge for longer data records, especially around vulnerable groups—kids, pregnant women, and those with allergies. Wastewater breakdown studies indicate that while the ester hydrolyzes naturally, the byproducts do not appear to accumulate like more problematic compounds. Environmental chemists keep watch, pushing for more studies on aquatic toxicity as usage volume climbs. Risk assessments translate these raw numbers into practical workplace and consumer guidelines.
Plasticizer markets feel the heat from governments and consumers pushing for low-VOC, low-toxicity alternatives. Adipate esters, and diisobutyl adipate in particular, stand to gain as legacy options get phased out. Researchers see opportunity in sourcing fully renewable feedstocks, even working to reduce water and energy use during manufacturing. In the short term, fields like consumer packaging, toys, and personal care show steady demand, anchored by regulatory acceptance and a history of safe use.
New uses appear on the horizon—flexible solar panels, greener sealants, smart wearable coatings. Research teams look for bio-based versions to close the loop on sustainability pledges. Real innovation shows up in supply-chain upgrades, like digital tags for traceability and batch integrity, helping everyone from producer to retailer to end user keep tabs on quality and safety. The shift toward conscious consumption and tougher regulations could keep pushing diisobutyl adipate to the top of the list, especially if green chemistry manages prices and performance. Industry players will need to keep eyes open, balancing classic chemistry workhorses with demands for cleaner, smarter, and safer materials.
Diisobutyl adipate sounds like one of those intimidating chemical names, but most folks run into it more than they realize. It’s a clear, odorless liquid, one often tucked away in ingredient lists of pretty normal things. Companies turn to it as a plasticizer, meaning it keeps products flexible and smooth. You’ll see it helping to soften plastics or playing an unsung part in the lotions and sunscreens sitting on bathroom shelves across the country.
Stepping into a store, look at plastic wraps, toys, shoe soles, and even synthetic leather purses—the touchable, bendy quality comes in large part from chemicals like diisobutyl adipate. The food industry relies on plastic films to keep produce and snacks fresh, and this compound helps those films keep flexibility. Take it out of the mix, and you’d end up with stiff, cracked packaging and bottles that break in your hands during cold weather.
Cosmetics is where many folks rub shoulders with diisobutyl adipate without a second thought. Sunscreens, moisturizers, and certain hair products rely on it. It helps these concoctions spread smoothly without feeling greasy or sticky. Over the years, I’ve dealt with eczema, so the ease of use and gentle feel in a cream means a lot. Dermatologists appreciate it, too, since it doesn’t clog pores or leave residue.
Whenever chemicals wind up in products people use directly on their bodies, safety conversations emerge quickly. Between government guidelines and company scrutiny, diisobutyl adipate comes under plenty of review. Scientific panels in the US and Europe have set concentration limits and checked for toxicity. So far, typical uses in cosmetics and packaging prove safe for regular exposure, with only rare reports of mild irritation or allergy.
But it’s hard to ignore the increased scrutiny over plasticizers in general, as stories about phthalates and endocrine disruptors make waves. Research on diisobutyl adipate hasn’t uncovered alarming effects at the levels used, but its long name sometimes triggers the same kind of public worry as other, less safe plasticizers. Ongoing independent studies spare no effort to keep these worries in check, which seems wise given how often folks encounter this chemical through skin or food contact.
The push for safer, greener chemistry can’t be ignored, especially with plastics and personal care. Biomaterials and naturally based plasticizers are gaining ground, spurred by stricter regulations and more informed consumers. Some companies now explore plasticizers sourced from vegetable oils or other renewables, hoping to keep the same feel and function as diisobutyl adipate.
From my own experience tinkering with homemade lotions, every tweak to a formula—especially removing well-tested ingredients—shifts how it feels and blends. For large brands, a shift to alternative plasticizers takes more than wishful thinking. They juggle cost, performance, and safety, all under the eyes of regulators and shoppers. Progress continues to move forward, with researchers adapting and testing new blends that balance user safety with environmental care.
Customers have more information now than ever. Reading labels, checking resources like the Environmental Working Group, and asking questions at the pharmacy now comes as second nature. Diisobutyl adipate stands as one of the many chemicals managed by watchful eyes and updated standards. As science expands and more alternatives step up, folks keep the power to make smart choices for themselves and the world around them.
Diisobutyl adipate pops up on ingredient lists in sunscreens, lotions, creams, and makeup. Its job is simple enough: soften skin, help products spread, make things feel light instead of greasy. It doesn’t seem flashy at first glance, just a workhorse that helps cosmetics feel more pleasant. The rise in label-checking and conscious beauty shopping means more people notice names like this, so it’s normal to feel some concern. I’ve seen confusion in forums and among friends about it—anything that sounds synthetic usually raises a few eyebrows.
Years ago, my friend got a rash that sent her on a long journey decoding every single chemical in her moisturizer. Most reports point to Diisobutyl adipate as a low-irritation risk for most people. Cosmetic Ingredient Review (CIR) scientists have evaluated its safety and green-lighted its use in cosmetics, with typical concentrations ranging between 0.5% and 8%. The European Union, a region with strict cosmetic standards, allows its use in personal care products.
Large-scale studies show low skin irritation risk for those without underlying skin conditions. It's not considered a proven sensitizer, either. Dermal toxicity studies in animals, cited in regulatory filings, found even large doses didn’t cause long-term harm. My own experience echoes the science: people who use reputable sunscreen or moisturizer brands containing this ingredient rarely report burning or itching directly linked to it. Allergists and dermatologists focus far more on widely known triggers like fragrances, preservatives, or certain plant extracts.
Industry data and public studies show Diisobutyl adipate doesn’t accumulate in the body. Human skin doesn’t tend to absorb large amounts of it, and it’s readily metabolized. On a practical level, I’ve found that contact reactions tie into other ingredients far more often. Still, there are those with especially sensitive or damaged skin who may develop mild irritation. No chemical ingredient is completely risk-free. Patch testing new products remains wise for anyone with allergies or recent skin issues.
Some people raise questions about environmental and long-term health effects. Many cosmetic chemicals, especially plasticizers and emollients, face ongoing review. The environmental picture is an evolving discussion, especially for compounds that can persist in water supplies. For people concerned with eco-friendly choices, picking biodegradable or plant-based ingredients might offer peace of mind, though these options also deserve close study.
Transparency matters, and companies have responded with better labeling and clear customer information. If you’re shopping for a gentle, non-greasy sunscreen or moisturizer and see Diisobutyl adipate, know that most dermatology sources, scientific studies, and regulatory groups agree—it ranks low on the risk scale. Checking for certifications or dermatological testing builds even more trust.
For those who want to avoid this ingredient, alternatives like caprylic/capric triglyceride or various plant oils exist. These swap-ins fit into formulas just as easily. Ingredient preferences should always reflect your own comfort and health priorities. I encourage people to test for themselves, read labels carefully, and talk to a dermatologist if unexplained reactions appear. Taking control of your own skincare feels less daunting than it used to, thanks to a deeper pool of real-world experience and scientific support available to everyday shoppers.
Diisobutyl adipate usually shows up as a clear, almost colorless liquid. I’ve noticed that it doesn’t bring any visible fuss to a workspace, unlike some chemicals that can startle you with their color or stench. There's a faint, barely-there odor—almost bland, hardly noticeable in small spaces. Many folks working around it for years hardly notice anything more than a hint of fruitiness, which often gets drowned out by stronger smells from other ingredients around.
Diisobutyl adipate dissolves in many organic solvents. It mixes especially well with oils and esters commonly used in cosmetics and topical products. Just try running a simple mixing test in the lab: blend a bit of diisobutyl adipate with castor oil or mineral oil, and it turns out smooth, no awkward separation. Water gives it trouble. Pour some in a beaker of water and watch the two layers form. No matter how much shaking, oil stays stubbornly apart. This property gives formulators less trouble when they build solutions meant for lips and skin, not drinks.
Poured into your palm or onto glass, diisobutyl adipate flows fast—low viscosity does the work. This thin, slick feel comes in handy for makeup, sunscreen, and skin-care creams. In the real world, it cuts the greasy texture people dislike in oily creams. Pick up a bottle, tip it, and it slides right out. Compare it to heavier esters, and diisobutyl adipate runs with a lightness almost like vegetable oil, never the weight you get from castor or lanolin.
Using the numbers found in chemical safety data sheets, diisobutyl adipate boils at about 320°C. Dropping it into a fridge or freezer doesn't turn it solid—its freezing point lands well under -50°C, so normal home or lab storage keeps it ready to pour. This broad temperature range lets factories and pharmacies use it from cold rooms to heated production lines without nasty surprises like sudden crystallization or boiling over. In my experience, you rarely have to babysit the containers, even if the AC goes out or things heat up during summer delivery runs.
Flash point often goes overlooked until something goes wrong. Diisobutyl adipate usually won’t spark up unless it hits around 170°C. Most users never get close to this temperature, but it’s still smart to keep it away from open flames or hot machinery. Those running bulk vats tend to stash it far from burners, and always have a spill kit and good ventilation—common sense keeps accidents down.
For cosmetic chemists and industrial users, these physical traits shape how the ingredient works and stays safe. Diisobutyl adipate’s ease of mixing, low odor, fast pour, and thermal stability mean less downtime, fewer bad batches, and products that glide on smoothly. The numbers aren’t just trivia; they matter every day in the lab, on the filling line, and for anyone wearing the final lotion or ointment. Blindly swapping ingredients based only on price or availability can wreck a formula’s feel or create hazards, so knowing these basic facts protects both worker and customer. Smarter choices, grounded in real details, build better products and keep trouble out of the headlines.
Diisobutyl adipate pops up in all sorts of products—sun care lotions, plastics, some adhesives. Folks checking ingredient lists wonder about more than just safety on skin; many want to know what happens after this chemical leaves their lives. Is it hanging around in the environment, or does it break down? This sort of question draws attention because the chemicals we use stick around far longer than we do.
Once, cleaning up after a backyard barbecue, I found bits of plastic still hiding under the grass months later. The lesson was clear: out of sight doesn’t mean gone. It’s the same story chemicals tell. Just because something slips down the drain doesn’t mean it’s disappeared from the planet. So, what’s the real story for diisobutyl adipate?
Research shows diisobutyl adipate lands in the “readily biodegradable” category. In composting tests and wastewater treatment studies, it tends to break down fairly quickly—much faster than old-school phthalates or other plasticizers clogging up streams for decades. A 28-day OECD test found that over 60% of diisobutyl adipate broke down, putting it above the typical bar for calling something biodegradable. Microbes can use the carbon inside it as food, transforming the chemical into simpler forms like water and carbon dioxide.
Biodegradable sounds great, but there’s real-world messiness to sort out. My experience gardening taught me that mulch labeled “biodegradable” could take years to break down outside a controlled compost bin. It's no stretch to imagine industrial chemicals behave the same way. If the soil stays cold or has the wrong bugs, something that would break down in a lab sometimes sticks around.
Breakdown speed also depends on where the chemical goes. Inside a sewage plant with lots of bacteria and oxygen, diisobutyl adipate vanishes quicker than out in dry soil or groundwater. It can lag in places without the right mix of life and warmth. Dumping anything in great volumes, even biodegradable stuff, risks swamping systems that normally handle these pollutants.
Most experts agree this chemical doesn’t build up in living things or cause big headaches for water creatures. Reviews from agencies like the European Chemicals Agency back this up, saying it’s got “low bioaccumulation potential.” It leaves the water, gets eaten by bacteria, and doesn’t persist like old-school pollutants.
That doesn’t give license to take the easy path. History tells us that products considered harmless can still cause problems if nobody checks disposal habits. Regulations nudge companies to keep their emissions low, but gaps always exist. Sometimes industries race to adopt seemingly greener alternatives without thinking about all the steps. Tighter rules around plasticizers—including third-party audits and consumer transparency—matter more than ever.
For anyone serious about reducing pollution, environmental claims on labels only matter when backed by data. The biodegradability of diisobutyl adipate sends a positive signal, but nobody should treat that as automatic permission for business as usual. Swapping in natural ingredients, using less packaging, and improving disposal practices work together to keep waterways clean. A product breaking down in weeks instead of decades makes a difference—yet good stewardship demands more than just checking the “biodegradable” box.
Walking through store aisles packed with creams, shampoos, and household items, a name like diisobutyl adipate often hides in fine print. Years of working closely with product development teams taught me how much this chemical crops up in daily-use products. It pops up in more ways than most realize, touching lives even if the name seems unfamiliar.
Diisobutyl adipate shows up in lotions, sunscreens, and makeup. Skincare manufacturers reach for it because it feels smooth, doesn’t feel greasy, and helps products glide across skin. This ingredient helps sunscreen formulas absorb without leaving a heavy white cast, which remains a big ask among people with darker skin or oily complexions. It also helps slow down water loss from the skin, which lets moisturizers work harder for longer stretches through the day.
Cosmetic scientists have found that diisobutyl adipate doesn’t cause breakouts for most people and has a low risk of irritation. That goes a long way in building trust in the beauty aisle, especially as many consumers read labels and watch for anything that might clog pores. With the global skincare market topping hundreds of billions, consumer safety and comfort drives ingredient selection. In these spaces, well-reviewed chemicals matter for both well-being and product sales.
Plastic manufacturers often look for ways to make their products soft and flexible. I’ve seen how shoe soles, swimming pool liners, and even car interiors rely on plasticizers to deliver that bend or give. Diisobutyl adipate turns up as a plasticizer in many products made with polyvinyl chloride (PVC). Instead of materials snapping under pressure, products hold up to daily use. Kids’ toys and flooring materials lean on this flexibility too, demanding chemicals with proven safety records and restricted toxicity.
The need for safer plasticizers grew after concerns about phthalates, which pushed manufacturers to seek alternatives. Diisobutyl adipate fits here as a lower-risk option, confirmed by studies showing that it breaks down in the environment with less risk compared to older choices. As regulators keep a closer watch on child safety and chemical migration, using established alternatives helps companies avoid both recalls and fines.
Machinery runs smoothly not only because of engineering but also the right blend of additives in oils and greases. During my time touring manufacturing plants, diisobutyl adipate cropped up in some high-performance lubricants and fluids. It helps cut friction between moving parts, keeps oil from gumming up in the cold, and doesn’t cause rust.
In paints and coatings, diisobutyl adipate helps solvents spread pigment evenly across surfaces. Contractors and DIY painters benefit from this because it makes application less frustrating and helps avoid streaks or patchiness—especially on large surfaces like walls or cars. An even coat matters if the goal is durability and appearance.
The chemical industry faces growing calls for more sustainable and less toxic ingredients. As a regular participant in product safety reviews, I've seen firsthand how companies vet additives like diisobutyl adipate for health impact, environmental break-down, and regulatory compliance. Pushing for more research and transparency allows consumers to make informed choices while producers stay ahead of shifting legal standards.
Trust gets built product by product, ingredient by ingredient. A focus on continuous improvement, honest labeling, and active engagement with scientific advances has the power to raise industry standards—making daily essentials both safer and better performing for everyone who relies on them.
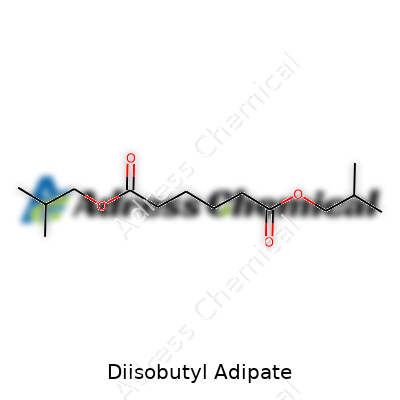
| Names | |
| Preferred IUPAC name | bis(2-methylpropyl) hexanedioate |
| Other names |
Adipic acid diisobutyl ester DIBA Diisobutyl hexanedioate Hexanedioic acid, diisobutyl ester |
| Pronunciation | /daɪˌaɪsəˈbjuːtɪl ˈædɪpeɪt/ |
| Identifiers | |
| CAS Number | 141-04-8 |
| Beilstein Reference | 1720893 |
| ChEBI | CHEBI:88834 |
| ChEMBL | CHEMBL1697828 |
| ChemSpider | 16688 |
| DrugBank | DB14170 |
| ECHA InfoCard | ECHA InfoCard: 100.009.676 |
| EC Number | 203-090-1 |
| Gmelin Reference | 83897 |
| KEGG | C19746 |
| MeSH | D02.241.081.700.274.262 |
| PubChem CID | 7516 |
| RTECS number | AU8400000 |
| UNII | K2HFX9C7L4 |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID7020162 |
| Properties | |
| Chemical formula | C14H26O4 |
| Molar mass | 286.44 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.95 g/cm3 |
| Solubility in water | Insoluble |
| log P | 3.9 |
| Vapor pressure | 0.02 mmHg (20°C) |
| Acidity (pKa) | 9.72 |
| Basicity (pKb) | > 12.42 |
| Magnetic susceptibility (χ) | -8.87e-6 |
| Refractive index (nD) | 1.4340 |
| Viscosity | 13.5 mPa·s (25°C) |
| Dipole moment | 2.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 354.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -980.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –4015.6 kJ/mol |
| Pharmacology | |
| ATC code | D11AX20 |
| Hazards | |
| Main hazards | May cause eye and skin irritation |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 171 °C |
| Autoignition temperature | 355°C |
| Lethal dose or concentration | LD50 oral rat 14,400 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 9,200 mg/kg |
| NIOSH | NA1570000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Diisobutyl Adipate: Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Dibutyl adipate Diethyl adipate Dimethyl adipate Diisobutyl phthalate Adipic acid |