Diethylene Glycol Monoethyl Ether, recognized by chemists for decades, has quietly fueled industrial growth since the early days of widespread solvents. Its roots stretch back into the twentieth century’s chemical breakthroughs, when researchers dug for alternatives with better solubility, less volatility, and lower toxicity compared to the harsher ethers and glycols in use. As industry expanded, new cleaning agents, coatings, and specialty products demanded solutions that could blend worlds—being gentle with equipment and the environment while still delivering results. It was this kind of chemical flexibility that helped Diethylene Glycol Monoethyl Ether step out of the lab and into the world of mass manufacturing.
Walk into any decent lab or paint manufacturing plant and a jug of Diethylene Glycol Monoethyl Ether is likely sitting on a shelf. Known by names such as Ethoxy Diglycol, Carbitol, Transcutol, or its chemical shorthand DEGMEE, the liquid stands out because of its faint odor, clear appearance, and surprising compatibility with both water and oils. Companies use it in artisan applications just as often as they do in big factories, tucked into formulas that need a helping hand getting things to dissolve, spread, mix, or penetrate surfaces.
Pour the liquid and it runs smoother than water, with a viscosity that helps it carry ingredients wherever it’s needed. Its boiling point lands well above standard room temperatures, which means it survives most processes without turning to vapor. Despite its industrial roots, DEGMEE doesn’t corrode containers or neighboring materials as some sharper chemicals do. For engineers, these basics mean more freedom to design safer workplaces and avoid hazards tied to evaporation and flammability. Its chemical structure—a glycol ether with an ethoxy group—gives it an edge blending polar and nonpolar substances, which opens the door to formulations traditional solvents simply can’t manage.
On the technical side, anyone picking up a drum can find the numbers: purity levels above 99%, a molecular weight of 134.17 g/mol, and a density hovering near 0.99 g/cm³ at room temperature. Labels flag its UN number (UN 1993), note its status as a combustible liquid, and warn about skin and eye irritation. Industrial suppliers must document all these details clearly—labeling isn’t just paperwork; it’s part of the safety line that keeps operators out of the hospital. Product codes may change between makers, but safety sheets—MSDS—set out the same core warnings. The right gloves, goggles, and ventilation gear all matter just as much as the chemical itself.
Most industry pros know it as a product of ethylene oxide reacting with ethyl alcohol, followed by selective distillation that weeds out unwanted byproducts. The process leans on tight controls—small temperature missteps lead to impurities or danger. Chemical plants run continuous or batch processes, each with their preference for catalyzers and purity targets. The goal is always maximizing yield, minimizing waste, and steering clear of runaway reactions. Recovered energy and careful recycling of process solvents help keep costs and environmental pressures down. The way DEGMEE is made shows how modern chemical engineering works in practice—scientists refine each step, not just for the sake of chemistry, but to deliver a liquid that meets practical, real-world needs.
In the lab, DEGMEE acts as both a team player and a wildcard. Mixed with acids, bases, or oxidizers, it obeys predictable rules but needs respect—overheating or bad combinations still hold risk. It plays well as a medium for organic reactions or as a launching pad for further modifications, thanks to its ether and alcohol functional groups. In coatings, it acts as a carrier, stretching drying times and making paints and inks more workable. Chemical engineers rely on its stability but openly debate which combinations make for the safest and most effective products, and push for processes that recover unused or spent material to cut environmental impact.
DEGMEE wears plenty of hats. Some labs call it Ethyl Carbitol, others rely on trade names like Transcutol or Carbitol Cellosolve (especially in pharmaceuticals). Catalogs may also list it as Diglycol Monoethyl Ether or 2-(2-Ethoxyethoxy)ethanol, a mouthful that chemists translate into product codes for easier handling. This sometimes confuses newcomers, especially when shifting between sectors—from pharma to ink manufacturing to textiles each region seems to have their preferred nickname, so cross-referencing is a daily routine for anyone dealing with logistics or purchasing.
Even though DEGMEE isn’t the worst offender in the chemical cupboard, respect for its hazards stands as the unwritten rule. Prolonged contact or poor ventilation can irritate the skin, eyes, and respiratory system. Regulatory limits like OSHA guidelines set ceilings for airborne concentrations—factory managers install extraction fans, hand out gloves, and run regular safety drills for good reason. My own work in a small-scale coatings plant showed me how often spill kits and emergency showers make a difference. Training isn’t just about box-ticking, and the best teams blend practical knowledge with clear emergency plans. As societies demand tighter workplace safety, DEGMEE remains in the “yellow caution” band—harmless if respected, unforgiving if ignored.
Everyday products use DEGMEE in ways most people never notice. Water-borne paints take advantage of its power to dissolve tricky pigments and allow for smoother application. Cleaning products need it to lift oily stains from hard surfaces. Pharmaceutical companies use it as a carrier solvent in topical solutions, helping drugs reach their target under the skin. Metalworking plants use it as a degreaser or flux remover. Even personal care products—perfumes, lotions—lean on it to carry fragrances and stabilize emulsions. Its reach is both broad and quietly vital, letting manufacturers deliver products with fewer harsh chemicals and better end results.
Research teams look to DEGMEE for more than dilution or cleaning. New coatings need it to help bind polymers for longer-lasting paints and corrosion-resistant films. Embedded sensor materials rely on its ability to mix with diverse chemicals and form stable solutions. Green chemistry researchers experiment with renewable resources for its production, aiming to cut down reliance on petrochemicals. Medical science pushes for safer drug delivery vehicles—studying how DEGMEE interacts with human tissue and how it can carry active ingredients more effectively, or adapt to targeted delivery techniques. The future for R&D hinges on understanding the fine line between performance and safety, and plenty of labs keep chipping away at optimizing chemical recipes and reducing unwanted side effects for the end user.
No solvent gets a free pass, and DEGMEE is no exception. Ingesting or inhaling enough can damage the liver, kidneys, or nervous system. Animal studies over the years have helped set workplace and consumer guidelines, showing exactly what overexposure does inside the body. In real-world terms, chemical spills, poor storage, or lack of protective gear lead to accidents. Regulatory agencies, especially in Europe and North America, constantly revisit safety data—tightening rules and rolling out new training programs. Lessons from past mistakes—like widespread glycol ether poisonings in the twentieth century—echo through modern safety standards. Responsible users read risk sections first, not last, before bringing DEGMEE into new processes.
Looking ahead, DEGMEE won’t disappear but it won’t stay static either. Pressure grows for bio-based solvents, recycling of waste streams, and final products with lower ecological footprints. Firms invest in greener synthesis routes, finding ways to use less energy and generate less hazardous waste. Product developers dig for replacements in sensitive applications, especially those involving children or consumer products with direct skin contact. Innovations in process control—using sensors and real-time monitoring—point to tighter oversight and fewer accidental exposures. As the demand for tougher environmental and health standards creeps up, the industry’s role shifts from simply supplying a reliable chemical to keeping workers, consumers, and ecosystems safer with every use. It’s a challenging road, but one with clear incentives for both innovation and caution—a story that’s still being written, every day, on the floor of chemical plants and research labs around the world.
Diethylene glycol monoethyl ether sounds like something out of a chemistry textbook, but this liquid touches so many areas of life. Looking at labels on household cleaners or industrial products, you might spot this tongue-twister. Its use stretches far beyond the lab, showing up in ways most people don’t notice. The reason boils down to its remarkable ability to dissolve and blend. This property gives it a front-row seat in making products more effective and safer to use.
You open a bottle of window cleaner, and the spray glides easily over the glass, picking up smudges and leaving things streak-free. That smooth experience often comes from solvents like diethylene glycol monoethyl ether. It can break down greasy residue and help cleaning agents spread evenly. Similar ingredients are tucked into paints and coatings. They stop the paint from drying too quickly, letting anyone get a more even finish, especially in tricky climates. Anyone who has rolled a coat of wall paint on a humid day will see the difference.
Shampoos and lotions owe part of their gentle feel and consistency to this chemical. It's chosen because it mixes well with water and oils. That means it helps deliver the benefits of conditioners right where they’re needed, smoothing out the mix and giving that rich, creamy touch. It softens the texture of skincare balms and helps fragrances dissolve, making perfumes last a bit longer on the skin. For people with sensitive skin, safer blends also depend on how well these types of solvents replace harsher ones.
Labs and factories turn to this chemical for its reliability. It keeps ink flowing in printers and in ballpoint pens without clogging. It shows up in industrial cleaners, stripping oils and stubborn stains off metal without leaving toxic residues. In the pharmaceutical world, it acts as a carrier, helping some medicines dissolve into liquids or gels. Drug designers need this level of versatility for both research and large-scale production.
No chemical comes without its worries. Some people hear a long name and wonder about safety. Large spills or repeated exposure in factories has brought up health concerns in the past, mostly through inhalation or skin contact. Regulations now set strict limits. Companies follow steps to cut the risk for workers and consumers. That’s why safety data sheets exist, spelling out how to use and store solvents like this. At home, the amounts found in products are usually tiny, but it makes sense to store cleaners out of reach of kids and to use them with common sense.
Sometimes, old habits die hard in manufacturing. Yet, demand for greener chemistry keeps pushing progress. Researchers hunt for plant-based or less toxic replacements. Some newer cleaning formulas cut down on synthetic solvents, easing the burden on the environment and workers. Until those alternatives work as well (and remain affordable), diethylene glycol monoethyl ether keeps its place in countless bottles under the sink and on factory shelves. Real progress comes from smart regulation, honest labeling, and persistent research into safer options.
Diethylene glycol monoethyl ether usually turns up in the world of solvents and cleaning agents, and some folks might notice it on labels for paints, inks, or even cosmetics. The long name hides the fact that this colorless, almost scentless liquid gets used because it easily mixes with water and dissolves greasy stuff. People rarely hear about the risks since most users trust manufacturers to sort out the safety part, but the story's not that simple.
Some basic chemistry classwork probably skipped over the effects of chemicals like this. Diethylene glycol monoethyl ether gets into the body through skin, lungs, or mouth. Without proper care, repeated or high exposures bring risks.
Chronic skin contact can leave behind everything from rashes to more serious reactions in people who already have sensitive skin. Breathing in vapors leads to irritation in throats and noses, almost like a persistent cold that won't clear up. Swallowing even moderate amounts causes stomach pain, vomiting, and can damage kidneys over time. In severe accidental poisonings, this stuff knocked out red blood cells, caused confusion, and pushed organs toward failure, according to medical case studies published in journals over the past decade.
Some animal studies have raised flags about long-term reproductive and developmental problems, pushing regulatory groups to label this as a possible risk for workers handling larger quantities. While many household products likely contain very small amounts, factory settings and cleaning industries have proven more dangerous. My own time working near chemical storage tanks in a factory reminded me how easy it is to underestimate fumes, even with fans running.
Most folks trust that government standards keep them safe, but cutting corners still happens. Cheap imports sometimes miss checks. I've heard stories from painters and janitors who didn't get gloves or masks, even as their workplaces demanded the cheapest bulk cleaning supplies. Short-term savings quickly get ugly when health costs add up or someone finally links recurring headaches to the chemicals around them. Workers might accept risk out of necessity, not choice.
Some people also risk exposure at home, thanks to poor ventilation or by mixing cleaning agents without reading labels. The hazards might seem remote until someone develops skin problems or breathing issues that just won’t go away.
Simple steps like checking safety data sheets before buying any cleaning supplies or paint can help people stay aware of what they’re breathing or touching. Employers owe their staff gloves, eye protection, and proper training so risks drop fast. Keeping chemical containers tightly sealed, storing them away from living spaces, and running extractor fans indoors make a huge difference.
On a wider scale, governments and regulators can keep pushing for clearer labels and tighter bans on excessive use in consumer products. Community groups sometimes organize workshops on chemical safety—these help people make sense of technical jargon and bring real know-how to neighborhoods. Class action lawsuits over chemical exposures have forced more companies to take transparency seriously, which shows that ordinary folks really can have an impact.
Chemicals aren’t spooky science fiction, but they deserve respect. Knowledge, a bit of caution, and teamwork between manufacturers, workers, and families can limit the risks and avoid a world of trouble from a single unassuming bottle.
In many factories and labs, Diethylene Glycol Monoethyl Ether pops up on supply lists. This stuff can help dissolve things, clean machinery, and even pop up in products like paints or inks. Workers sometimes treat it like just another clear liquid, but a misstep in storing or moving it leads straight to trouble. Even smaller leaks or exposure events have a knack for ruining a good day.
People working with Diethylene Glycol Monoethyl Ether will tell you, heat and sunlight don’t play nice with this solvent. I’ve seen containers left in a hot storeroom sweating and bulging, making everyone around nervous. Cool, shaded storage keeps this solvent stable. Don’t park drums near boilers or south-facing windows. A dry place, with steady temperatures under 25°C, buys peace of mind.
Drums need tight lids and good seals. Once, a leaky drum turned a storage closet into a slippery mess that took half a day to clean. Even “just a little” exposure isn’t worth it. If workers transfer this solvent into other containers, they should use ones made of stainless steel or certain plastics, like polyethylene, because some metals just corrode or react.
Ventilation isn’t something to cut corners on, either. I still remember the sharp, sweet-ish smell from a storeroom that’d been closed too long. It leaves a headache behind, and a long-term whiff can do worse, targeting livers and kidneys with steady exposure. Fans or exhaust systems make all the difference.
Pouring or pumping Diethylene Glycol Monoethyl Ether without gloves or goggles is asking for burns or bad skin rashes. I messed up once, skipping gloves for what I thought was a quick transfer, and dealt with itchy, red skin for days. Heavy-duty gloves, goggles, splash aprons, and sometimes respirators belong on the gear list.
Anyone working with this solvent should know the emergency drill. Even a careful person can spill or splash, so keeping eye-wash stations and emergency showers nearby isn’t just a formality. Spills spread fast. The right absorbent materials—like sand or commercial pads—can stop it from reaching floor drains or wider work areas.
Mixing Diethylene Glycol Monoethyl Ether with strong oxidizers throws open the door to violent chemical reactions. It's not just frustrating equipment damage—fire and gas risks jump up. Stores and work surfaces stay safer by keeping cleaning acids, peroxides, or strong bleaches far from the main drum. Color-coded shelves or labels help keep things where they belong.
Rules printed on a wall usually mean little without buy-in from the team. In shops where safety has stuck, workers swap stories about near-misses, and new hires get proper walkthroughs. Training needs hands-on time—not videos watched in the back room. Giving workers the right tools for safe transfer (pumps that don’t leak, containers built for the job) drops risk with every batch handled.
Regular safety drills and checks may slow things down for a day but prevent real accidents. Many factories run audits every few months, walking storage spaces and double-checking safety gear. Even the best labels wear off or containers degrade, so a quick scan of drums and equipment keeps everything one step ahead.
If you work with Diethylene Glycol Monoethyl Ether, storing and handling it with respect isn’t about extra paperwork—it saves you from ruined gear, wasted time, and health problems that don’t show up right away but hurt the most in the long run.
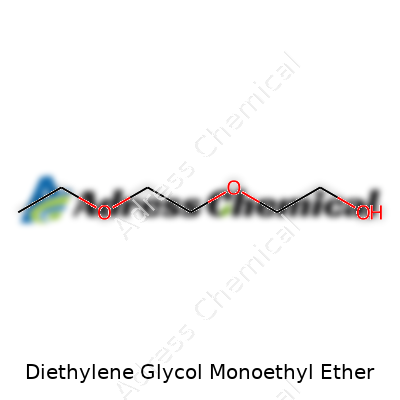
Some chemicals go by names that sound straight out of a science fiction novel. Diethylene glycol monoethyl ether fits that mold, but it's no mysterious brew. In the real world, many folks use it without even realizing. The chemical formula looks like this: C6H14O3. The structure adds a few more details—a chain built from two ethylene glycol units connected by an oxygen atom, capped off on one end by an ethyl group.
If you like facts, here’s one: it's got six carbons, fourteen hydrogens, and three oxygens. The way these pieces snap together changes how it acts. Picture two ethylene glycol parts (that’s -CH2CH2O- twice), then imagine an ethyl group (-CH2CH3) hanging from one end. Put it all together and you get: CH3CH2OCH2CH2OCH2CH2OH. What breaks this down is a simple backbone, but the oxygen atoms are spaced out just enough to let the molecule mix nicely with both oily and watery substances.
Anyone who's ever worked with solvents knows how picky some formulas can be. In my early days around a print shop, I spent hours cleaning ink with a cocktail of chemicals. Solvents like diethylene glycol monoethyl ether—sometimes called "DEGEE"—made the job easier. They don't just dissolve ink; they pull up old grease and leave machinery looking new. From a practical perspective, that’s gold.
People paint walls and clean up with different expectations. No one wants to deal with streaky layers or sticky remains. That’s where DEGEE shines. Paint makers pick this solvent for its gentle touch and even evaporation. Ink runs evenly—no clumping—because of how smoothly DEGEE lets colors spread. This chemical doesn't stop at art supplies. In cleaning fluids and metalwork, it carries away stubborn gunk and leaves surfaces dry, not tacky.
With wide utility comes a responsibility. In small doses, DEGEE stays out of the headlines. It slips into fabric cleaners or acts as a carrier in pesticides. But accidents or careless handling bring risk. Swallowing even modest amounts sends folks to the ER with organ issues. So it’s no surprise that workplaces keep tight storage and solid training on deck.
My lab training hammered home one thing: a chemical is only as safe as the person handling it. Rushing a spill cleanup or skipping gloves can flip from routine to danger in a blink. Manufacturers and employers should make safety data easy to find, label every container, and offer regular drills—not as boxes to check, but as habits to save lives.
In production, shifting toward sealed systems and improved ventilation protects everyone around. On the consumer end, enforcing clear labels and perhaps adding less toxic alternatives for household products can spare headaches and worse. In places with fewer resources, sharing old-school wisdom and simple posters does far more than a rulebook gathering dust.
Every bottle with C6H14O3 on its label offers utility, but demands care. Understanding its structure isn’t just for chemists—anyone using it should know what it can do, and what it can undo if ignored. Real solutions don’t come from lecture halls. They grow out of the ordinary moments where someone chooses to read that label, grab the gloves, or slow down just a beat before pouring.
Walk into any lab or production floor working with chemicals, and you notice there’s always a conversation between compatibility and speed. Diethylene Glycol Monoethyl Ether, a mouthful for sure, shows up in this back-and-forth constantly. The question that always pops up: does it actually get along with water, and what about other solvents?
Let’s keep things simple. Diethylene Glycol Monoethyl Ether, often labeled as DEGMEE or DEGEE on drum stickers, mixes right into water. Not a drop floating on the top, no weird layering that needs an industrial stirrer — these two become one. Now, that compatibility puts this chemical on the radar for folks making cleaning agents, water-based paints, and even pharmaceuticals. It isn’t fussy about water, but the story changes when you start throwing other solvents into the mix.
I’ve spent years in product development, so I’ve seen how easy it is to underestimate what happens when you pour two liquids together. If you pick the wrong partner, you end up with sludge, layer separation, and clogged equipment filters. That means costly downtime. With mixing, you want cooperation, not a war in the tank.
DEGMEE answers the call not only with water but with a handful of organic solvents as well. It dissolves well with alcohols like ethanol, methanol, and isopropanol. So if you’re in a processing plant switching between small-batch cleaning solutions and big runs of electronics coatings, you don’t need a new protocol or a chemist watching each batch like a hawk.
Take esters, ketones, and glycols. DEGMEE slides in with them too. Acetone, ethyl acetate, diethylene glycol itself — they all mix without trouble. These combinations mean formulators get flexibility. I’ve watched people push product performance up a notch just because this solvent handled the ‘mixing’ headaches, letting them focus on things like finish quality or drying time instead of chemical fights.
There’s a wall — and it’s oils. DEGMEE and mineral oils might look like they’ll play nice, but they keep their distance. You end up with stubborn layers. So in personal care or lubricants, this chemical steps aside. I’ve seen novice formulators waste hours shaking up emulsions that just won’t come together. You learn fast where the line is.
Anyone who’s handled solvents knows it pays to keep your wits about you. DEGMEE isn’t the harshest around, but skin and lung exposure won’t do anybody any favors. Proper gloves, goggles, and ventilation—non-negotiable. I’ve worked in places that cut corners and watched ambulance lights show up at the gate more than once. Safety isn’t overrated.
Today’s manufacturing needs keep shifting. More water-based, less toxic, friendlier to people and planet — customers demand it, regulators demand it. DEGMEE’s ability to run with water and a stable of other solvents keeps it in play as these trends accelerate. It keeps manufacturers agile, lets product designers respond quickly, and honestly, it just makes life easier when you’ve got a solvent that plays well across so many applications. That saves headaches and, ultimately, saves money.
| Names | |
| Preferred IUPAC name | 2-(2-ethoxyethoxy)ethan-1-ol |
| Other names |
Ethoxydiglycol DEGEE Diethylene glycol ethyl ether 2-(2-Ethoxyethoxy)ethanol Carbitol Transcutol |
| Pronunciation | /daɪˈɛθɪliːn ˈɡlaɪˌkɒl məˈnoʊˈɛθəl ˈiːθər/ |
| Identifiers | |
| CAS Number | 111-90-0 |
| Beilstein Reference | 1460220 |
| ChEBI | CHEBI:31587 |
| ChEMBL | CHEMBL1352 |
| ChemSpider | 6633 |
| DrugBank | DB06715 |
| ECHA InfoCard | 100.036.299 |
| EC Number | 203-919-7 |
| Gmelin Reference | 82280 |
| KEGG | C06510 |
| MeSH | D003994 |
| PubChem CID | 8177 |
| RTECS number | KK8225000 |
| UNII | 6IO9OI1ZBX |
| UN number | UN1171 |
| CompTox Dashboard (EPA) | DTXSID5023864 |
| Properties | |
| Chemical formula | C6H14O3 |
| Molar mass | 134.18 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Odorless |
| Density | 0.988 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.54 |
| Vapor pressure | 0.067 mmHg (20°C) |
| Acidity (pKa) | 14.78 |
| Basicity (pKb) | 15.4 |
| Magnetic susceptibility (χ) | -7.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 1.7 mPa·s (at 25°C) |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 252.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -664.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3970 kJ/mol |
| Pharmacology | |
| ATC code | D02AE12 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 94 °C |
| Autoignition temperature | 215 °C |
| Explosive limits | 3.6–23.0% |
| Lethal dose or concentration | LD50 (Oral, Rat): 6050 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2,500 mg/kg (rat, oral) |
| NIOSH | WI1100000 |
| PEL (Permissible) | 5 ppm |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Diethylene glycol Monoethylene glycol Triethylene glycol Ethylene glycol monoethyl ether Diethylene glycol monomethyl ether Diethylene glycol monoethyl ether acetate |