Talking about solvents, Diethylene Glycol Methyl Ether (DEGME)—sometimes seen under names like methyl carbitol or 2-(2-methoxyethoxy)ethanol—has been around since chemical companies ramped up production of synthetic additives in the early-to-mid 20th century. It started cropping up as the pull for specialized paints and coatings took off. Folks wanted liquids that dried slow, mixed well, and didn’t flare up with a spark. DEGME soon worked its way out of paint shops into cleaners, inks, and resins. Manufacturers saw its value each time older chemicals got flagged for health or flammability issues. The rise in demand follows how industries chased performance and safety—less explosive than regular ethers, better solvency than straight glycols, and easier to handle every step from factory to warehouse.
Contenders in the solvent family always battle for a mix of convenience, safety, and tack. DEGME found its lane here by balancing between volatility and strength. It brings a signature sweet odor, clear appearance, and a liquid form that pours easy at room temperature. Chemists in industries often reach for it in lacquers, cleaning formulas, printing inks, and select agricultural chemicals. That versatility doesn’t just spring out of nowhere; DEGME fits formulas that want to look good, last longer, and avoid bottle explosions or hazardous vapor clouds.
Look at DEGME on paper and you’ll find a boiling point sitting close to 194 degrees Celsius and a freezing point that keeps it liquid in most countries all year. With a molecular formula of C5H12O3 and a molecular weight around 120.15 g/mol, it dissolves in water almost as easily as sugar, mixing seamlessly with alcohols and other glycol ethers. On the safety side, the faint odor won’t bother most people, but careless storage near open flames can cause headaches. For environmental folks, its low vapor pressure means it escapes into the air at a slower pace than conventional solvents, so storage and ventilation decisions matter. Solubility stats win big for cleaning up messes—water washes it away, yet its affinity for oils helps remove sticky residues or polish surfaces until they sparkle.
Drums and tankers rolling into factories usually sport hazard symbols and the chemical’s CAS number: 111-77-3. Labels carry concentration info, batch numbers, and handling instructions that cut right to the chase: keep cool, keep shut, keep away from skin and eyes, and avoid mixing with oxidizers. Most industrial users pay close attention to purity percentages, as tiny impurities can change how a batch dries or cleans. Technical specs stress limits for moisture content, color index, and acid numbers. Safety Data Sheets (SDS) must travel with every shipment, giving employees a roadmap for dealing with spills, leaks, or accidental exposure.
DEGME rarely drops out of the sky as a simple byproduct. Factories cook it up by reacting diethylene glycol with methanol under acid catalysis—no complicated steps, but temperature and catalyst balance turns out cleaner, stronger solvent. After distillation pulls off water and heavier residues, chemical engineers test the final product for clue-in signs of leftover methanol or diethylene glycol. Getting a steady batch means keeping gear clean, temps stable, and raw chemicals at the right grade. Every percentage point of purity protects downstream users from odd odors or unexpected lumps in their paint or ink.
Chemists don’t just leave DEGME in its bucket—they tweak it. Under acid or base conditions, the ether can break apart or make new linkages with other alcohols and acids. That matters for companies designing resins or surfactants, where tight control over reactivity builds new products for adhesives, coatings, or even specialty cleaners. Some newer labs use DEGME as a starter for more complex glycol ethers, tailoring the molecule to do jobs DEGME alone can’t handle. Throw it into polymer reactions and it acts as a plasticizer, softening plastics or resins meant to last longer and withstand cracking under sunlight or cold.
Open up a catalog and DEGME might answer to methyl carbitol, 2-(2-methoxyethoxy)ethanol, or even DMM—depending on where it’s made or sold. Big chemical giants slap their own brand names to it, with Sigma-Aldrich, Dow, and BASF all claiming space on the labels. Anyone cross-referencing for safety or supply reasons needs to keep these aliases straight because a missed synonym can mean the wrong drum gets delivered or worse, the wrong safety gear gets picked for the job.
Once you deal with large drums of DEGME, training outpaces paperwork. Health risks come from skin absorption, inhalation, or splashes to the eyes. Workers suit up in gloves, goggles, sometimes even full-face shields, especially where splatter risk jumps up around mixers or pumps. Facility rules reinforce spill kits, shower stations, and constant ventilation—forgetting ventilation means a single mistake can pull someone off the shop floor with headaches, dizziness, or worse. Regulations from OSHA and comparable authorities lay down exposure limits. The American Conference of Governmental Industrial Hygienists (ACGIH) sets a Threshold Limit Value (TLV) of about 10 ppm for an 8-hour work shift—nothing to gamble with.
Factories use DEGME to thin water-based paints and inks, soften textile treatments, and tweak household cleaners looking for a balance between deep cleaning and gentleness to hands. In electronics, it finds purpose as a cleaning solvent that takes circuit board grime off without dissolving delicate parts. Farmers sometimes turn to it in pesticide formulations when other solvents wreck plant leaves or carry odors a mile downwind. Each sector values what DEGME can do that simple alcohols or acetone can’t match—slower evaporation, gentle solvency, and lower smog contribution.
Some research labs experiment with DEGME for biodegradable polymer development or as an additive in new-generation battery solutions. Specialty coatings developers play with different glycol ethers to optimize drying times or scratch resistance—DEGME offers a middle ground for tinkering, thanks to decades of test data. University research sometimes looks for safer alternatives, probing glycol ethers one by one for less toxicity or easier disposal, spurred by shifts in chemical regulations. Each finding builds on the old, trading away risk for better results in labs, factories, and eventually, consumer products.
Long-term studies make clear that overexposure to DEGME brings risk, especially for blood, kidney, and reproductive systems. Workers chronically breathing vapors or getting regular skin contact face higher odds of headaches, fatigue, irritation, and more serious chronic effects. Lab animal tests back this up, so medical researchers push for regular health check-ups and monitor biomarkers in high-risk workplaces. Lawsuits and regulatory changes over the decades reflect lessons learned—good ventilation and strict controls actually make the difference between safe and hazardous handling on the job.
Growth in coatings, adhesives, and specialty cleaning products keeps DEGME in circulation, but pressure mounts for less-toxic, eco-friendly upgrades as green chemistry takes hold. Companies innovate with new blends that swap out DEGME for safer glycol ethers, but those take time to prove their real-world performance. As regulations evolve in Europe, America, and Asia, suppliers hustle to stay ahead with reformulated products or improved purity grades. Up-and-coming tech—like flexible circuits, biodegradable plastics, or solvent-free adhesives—gives both challenge and opportunity, as new markets look for performance but not at the expense of health or environment. For now, experience shows knowledge, respect for risk, and a clear eye on safety shape how DEGME remains part of the industrial toolbox.
Folks don’t talk much about diethylene glycol methyl ether at backyard barbecues, but it touches a lot of everyday experiences. This chemical, tucked behind the abbreviation DGME or sometimes called methoxy diglycol, plays a key role in making certain products work as they should. Anyone who’s painted a room or cleaned a window with a store-bought cleaner probably interacted with this compound without realizing it.
Painters, printers, and manufacturers know this stuff for its ability to help things mix and spread. Companies turn to DGME for blending because it’s a strong solvent—meaning it can dissolve other substances pretty well. Latex paints, inks, and specialty coatings rely on it to keep everything smooth and prevent uneven drying. Walk by a construction crew rolling paint onto a storefront or a crew working with specialty glues and chances are some DGME helped make that job easier.
It has a way of stabilizing things that need to stay mixed. Think about cleaning fluids. Instead of separating into layers, window cleaners and similar products use DGME to mix water and oily agents together. The result: less streaking, and glass that actually looks clear. There’s science behind the scenes, with DGME making the solution both effective and easy to apply.
Some chemicals hang around because they’re cheap; others because nothing else quite does the job. DGME fits both profiles. Its chemical cousin, diethylene glycol, gets used in antifreeze and brakes, but DGME brings a slightly different punch. Thanks to its ability to blend water and oil, it doesn’t just stick to paint and cleaners. You’ll see it show up in the printing world, where it keeps ink flowing smoothly through press machines. It’s used when ink needs to dry slowly so pages don’t smudge—a familiar headache in any print shop.
It also pops up in electronics, usually as a solvent for specialty adhesives and coatings. Anyone building circuit boards at scale runs into challenges where wires and parts need protecting, and DGME steps in to deliver coatings that stay put and last longer than a quick-fix alternative. The tech world, despite pushing digital, runs on reliable compounds like this one.
Strong solvents bring real concerns. DGME isn’t as infamous as lead paint, but repeated overexposure at work can irritate skin and eyes. Breathing in fumes over long shifts around open barrels can spark health worries. News stories run with headlines about workplace safety for a reason—problems don’t just pop up in the lab or chemical plant. Small shops and garages with poor ventilation sometimes don’t realize the risk until employees start dealing with headaches or rashes.
Staying safe doesn’t require fancy tech. Good airflow, gloves, and updated safety sheets can cut most risk. Regulations say how much can hang around in the air, but enforcement only matters when people actually read the rules and gear up. Poisonings and accidents tend to happen not from the complex properties but from plain old neglect.
Some businesses hunt for greener, less risky solvents. The tricky part: substitutes often cost more or don’t quite deliver the same results. Water-based paints and cleaners are better for the planet, but switching brings headaches for folks used to old formulas. Change moves slowly in manufacturing, and the push for safer workplaces outpaces the launch of “green” solvents that actually work.
DGME sticks around because it works well and costs less than many alternatives. Until something fits those tracks, every shop and factory must keep a close eye on how they use and store it. Awareness and simple precautions often shape outcomes more than any label or warning sticker.
Opening a drum of Diethylene Glycol Methyl Ether, the sharp odor tells you quickly this isn’t something you want on your skin or in your lungs. I’ve seen experienced workers drop their guard only to end up in the emergency room with chemical burns. Many folks think gloves and goggles are more of a formality, but this solvent has a way of finding the smallest cuts on your hands, causing stinging and lasting skin irritation. Prolonged exposure brings headaches, nausea, and sometimes even more serious harm to your kidneys and liver.
No one likes wearing chemical-resistant gloves and a long-sleeved shirt on a hot day. After a coworker suffered a splash injury that left a painful rash and scarring, the lab crew at my old job stopped skipping steps out of convenience. Nitrile or neoprene gloves are a must, not those basic thin latex ones. Safety goggles protect eyes better than glasses, and for full protection, face shields are worth the slight hassle. Even one splash to the eye can mean months of recovery, and nobody wants that.
Most accidents happen because people underestimate fumes. In one cramped storage room, I once watched a friend get dizzy in less than ten minutes before realizing the extractor fan wasn’t working. Fume hoods or open windows with direct airflow make a huge difference, especially since this ether evaporates easily and fills the air with invisible vapors that mess up your head or worse. Setting up adequate ventilation is much simpler than trying to explain a chemical inhalation incident later.
I once heard about a fire in a corner store because someone stored solvents near a heat source. Diethylene Glycol Methyl Ether’s vapors catch a spark faster than most realize. Always keep the container tightly closed, away from sunlight and heat. Steel cabinets or designated chemical lockers help contain spills. Clear labels help everyone in the workplace recognize the hazard at a glance, saving crucial time during emergencies.
Every lab and workshop should keep a chemical spill kit handy. After one spill soaked through a colleague’s jeans, everyone became meticulous about keeping absorbent pads, neutralizers, and proper waste containers on hand. The right gear turns a small mess into a minor inconvenience instead of a health crisis. Absorb with vermiculite or another inert material, sweep it up carefully, and make sure no one gets it on their shoes or hands. Never let solvents go down the drain—treat the waste with respect.
I once onboarded a new tech who thought water would wash away chemical exposure. In reality, water might spread this solvent and make contact worse. Regular safety training saves more than just time; it helps spot risks before they turn into real problems. Safety isn’t about following rules for the sake of it—it’s about making sure everyone gets home safe, every night.
Experience teaches the hard way, but it doesn’t need to. If in doubt, check the Safety Data Sheet, talk with colleagues, and plan the work before starting. No shortcut or skipped step has ever been worth the price of irreversible harm. Whether you’re new to the field or have logged decades around chemicals, treating Diethylene Glycol Methyl Ether with respect goes a long way toward keeping yourself and everyone else out of harm’s way.
Diethylene glycol methyl ether, known among chemists as DEGME, carries the chemical formula C5H12O3. To break it down, it has five carbon atoms, twelve hydrogens and three oxygens. The structure isn't complicated; you’ve got two ethylene groups joined by oxygen, finished off with a methyl ether on the end. The formula connects more than elements—it links this colorless liquid to a surprising number of everyday products.
I've spent my share of hours mixing solvents in small, poorly ventilated rooms. DEGME shows up looking completely transparent, with almost no scent. It feels slippery on the skin, and it's heavier than water, with a density around 1.02 grams per cubic centimeter. Pour it into a beaker, and you notice how it doesn’t evaporate quickly. Its boiling point falls around 194°C (381°F), and it doesn’t freeze until temperatures drop to about -70°C (-94°F), making it useful where other solvents would lock up or boil off.
Unlike many common solvents, DEGME blends smoothly with water, alcohols, and even ether. That means you can use it to thin paints, inks, or mix up coatings without worrying about clumping or separation. This quality caught my attention years ago working in a print shop, where water-miscible solvents ended up saving a lot of time cleaning screens and presses. No need to reach for three different bottles—one would do the job.
The uses for DEGME stretch far beyond the laboratory. Paint manufacturers reach for this ether to adjust viscosity in water-based and solvent-based paints, so brushes glide across surfaces and coatings dry without streaking. Electronics factories add it to cleaning solutions for printed circuit boards; workers appreciate solvents that clean effectively without damaging sensitive traces or components.
My experience in the coatings industry showed me how DEGME’s even evaporation plays a big role in achieving a flawless finish. Solvents that evaporate too fast leave behind bubbles; slow ones can make surfaces sticky for days. DEGME finds a middle ground—just the right volatility so coatings level out smoothly before they harden.
On the downside, like many glycol ethers, DEGME doesn’t come without risks. I’ve watched safety teams monitor air levels, especially in enclosed spaces, since long exposure can irritate the skin, eyes, or lungs. Current research links heavy occupational contact to health effects, making gloves and ventilation more than bureaucratic boxes to check—they matter every day. Regulatory bodies in Europe and North America have issued guidance on exposure limits for precisely this reason.
The push for “greener chemistry” draws attention here. Many companies push for alternative solvents with lower toxicity profiles, but performance and cost can complicate switching away from DEGME. In my view, manufacturers need to stick with strong workplace training, frequent air checks, and robust personal protective equipment as long as DEGME stays on the ingredient list.
Cleaner technologies eventually find their way into the mainstream. Until then, knowing the details—formula, properties, workplace impacts—lets everyone from factory planners to DIY enthusiasts make smarter decisions. It’s clear you can’t approach solvents like DEGME casually; respect and good information go a long way.
Diethylene glycol methyl ether doesn’t usually make headlines, but those who’ve worked in chemical plants or research labs know this solvent by another name: a tricky liquid that demands some respect. Spills aren’t just messy—they’re risky to health and the environment. I’ve seen firsthand how cutting corners in chemical storage always comes back to bite, whether through vapor build-up or hurried cleanups with the wrong materials. People sometimes shrug off the warnings, but accidents don’t wait for you to read the manual.
Steel drums and high-density polyethylene containers handle this solvent well. Once, a facility where I consulted swapped in lightweight plastics to save money, not realizing solvent vapors eventually warped the seals. Months later, a faint but sour smell clued them in—by then, they needed to overhaul half their storage. Not every mistake ends that badly, but it’s a reminder that containers touch everything: cost, safety, and even insurance.
I’ve stepped into storage rooms that were more sauna than safety zone. Temperature control never feels urgent until someone sweats through a bottle seal or has fumes set off an alarm. Good airflow isn’t just about comfort; it keeps vapors from collecting at floor level, where they love to lurk. Degreasing shops and paint facilities sometimes forget this, and for years, low-level headaches or odd smells were the only clues before someone got sick. Good fans and a working thermostat make all the difference.
A storage room shouldn’t share walls with break rooms, welding stations, or fruit baskets—yet I’ve seen all three more than once. Putting solvents close to high-heat or eating areas just makes it easier for something to go wrong. Once, I visited a site where a makeshift storage closet sat ten feet from a microwaved lunch table. Folks just got used to the chemical sting in the air and wondered why their coffee tasted odd.
Even pros forget what’s in an unmarked jug after a week or two. I label every solvent, even if the bottle already says so, because people grab things in a rush or after a shift change. Accidents aren’t always about explosions or mystery illnesses; sometimes, they’re just a matter of someone pouring the wrong thing down a drain. Each labeled bottle saves questions and confusion.
During one summer internship, a half pint spilled along the warehouse floor. The manager waved it off, but hours later, the cleaning crew had mild headaches—nobody connected it to the spill. That experience sticks. Gloves and absorbents cost little compared to a hospital visit. Putting spill kits within arm’s reach, and reminding everyone how to use them, solves a lot more problems than waiting for a supervisor to handle it.
The best storage system falls apart if newcomers don’t know the rules. I always pushed for hands-on training, even for people who only handled solvents once a month. It feels like a hassle until the first time someone catches a whiff of fumes and actually knows where the fans and spill kit are stored. I’ve never regretted five extra minutes spent teaching the basics.
Diethylene glycol methyl ether doesn’t ring a bell for most people. If you’ve handled paint thinners, resins, or some inks, chances are you’ve brushed up against it. Companies reach for this solvent because it dissolves many things that water can’t touch. On paper, it does the job well — but that’s only one side of the story.
My first time working with industrial solvents, I remember walking into a lab, catching a sweet, chemical scent in the air. Back then, the safety talk barely scratched the surface. Years later, and with headlines about chemical exposures still popping up, it’s clear that talk should have gone deeper.
Breathing in vapors or getting this stuff on your skin can start causing problems your body won’t thank you for. Skin irritation creeps up at first, but that’s small potatoes compared to the headaches, dizziness, and fatigue that pop up with higher doses. Longer stretches around it can create even more trouble, affecting the liver or kidneys in ways that doctors are still piecing together. Animal research flags possible developmental effects — another red card worth noting for workplaces with loose controls.
One big problem comes from not seeing the risk. Diethylene glycol methyl ether isn’t as nasty-smelling as some of its cousins, so workers often let their guard down. Without proper gloves, clothes, or ventilation, that clear, sweet liquid can soak in fast, especially for folks on a busy line. According to some occupational safety databases, this solvent’s health threshold isn’t all that high. That tells me we’re not talking about a harmless tool.
Chemicals don’t stay put. Spills or sloppy disposal send diethylene glycol methyl ether down drains or onto soil. Out in the environment, it mixes with groundwater and doesn’t vanish overnight. Bacteria can break it down, but not fast enough to dodge contamination headlines. Drinking water and crops nearby take the hit. The EPA tracks this one because it doesn’t want another repeat of high-profile chemical leaks fouling up small-town water supplies.
There’s no rocket science in keeping people safe from solvents. Decades in labs and plant floors showed me that a few smart habits make the biggest difference. Wear gloves, keep workspaces vented, and store chemicals in airtight containers. The bigger victory comes from swapping in less toxic options. Some water-based or alcohol-based solvents handle the same jobs with fewer headaches — both for workers and the ecosystem.
Rules only go so far if people shrug them off. Supervisors need real buy-in, not just posted safety sheets. Walkthroughs to check gear, quick talks about spills, and rewards for clean habits turn rules into routines. On the environmental side, chemical companies could step up waste collection and make recycling solvents standard procedure. Research keeps pushing for green chemistry, cutting down both hazards and costs in the long run.
Slapping “hazardous” on a label is easy. Making sure that label means something takes effort from every side — workers, managers, regulators, and chemical makers. The payoff isn’t dry statistics; it’s seeing everyone get home safe and hearing fewer stories about sickened neighborhoods. Risk isn’t some abstract number. It’s what happens when we pretend a familiar solvent can’t hurt us — or the world outside our doors.
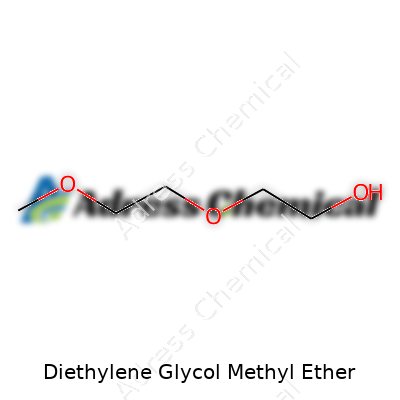
| Names | |
| Preferred IUPAC name | 2-methoxyethoxyethanol |
| Other names |
2-(2-Methoxyethoxy)ethanol DEGME Methyl Carbitol Diethylene glycol monomethyl ether Methoxy diglycol |
| Pronunciation | /daɪˈɛθ.ɪ.lin ˈɡlaɪ.kɒl ˈmiː.θəl ˈiː.θər/ |
| Identifiers | |
| CAS Number | 111-77-3 |
| Beilstein Reference | 1209375 |
| ChEBI | CHEBI:31587 |
| ChEMBL | CHEMBL1357 |
| ChemSpider | 5194 |
| DrugBank | DB14183 |
| ECHA InfoCard | 07b5f6d4-6792-4809-92f0-1bc8beccbd0f |
| EC Number | 203-906-6 |
| Gmelin Reference | 1520 |
| KEGG | C01447 |
| MeSH | D003994 |
| PubChem CID | 8163 |
| RTECS number | KL5950000 |
| UNII | KAE31012US |
| UN number | UN1161 |
| CompTox Dashboard (EPA) | DTXSID1020297 |
| Properties | |
| Chemical formula | C5H12O3 |
| Molar mass | 134.18 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.954 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.54 |
| Vapor pressure | 0.02 mmHg (20°C) |
| Acidity (pKa) | 14.8 |
| Basicity (pKb) | 0.40 |
| Magnetic susceptibility (χ) | -13.45×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.419 |
| Viscosity | 1.7 mPa·s (at 25°C) |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -674.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4103 kJ/mol |
| Pharmacology | |
| ATC code | D02AE08 |
| Hazards | |
| Main hazards | Harmful if swallowed, in contact with skin or if inhaled; causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 102 °C (216 °F) |
| Autoignition temperature | 215 °C (419 °F) |
| Explosive limits | Explosive limits: 1.5–19% |
| Lethal dose or concentration | LD50 (oral, rat): 6500 mg/kg |
| LD50 (median dose) | 5,130 mg/kg (rat, oral) |
| NIOSH | UB0150000 |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol methyl ether Diethylene glycol Diethylene glycol monoethyl ether Triethylene glycol monoethyl ether Ethylene glycol Propylene glycol methyl ether |