Diethylene Glycol Ethyl Ether Acetate traces its roots to the broader evolution of glycol ethers, which began shaking up chemistry labs around the 1930s. These solvents earned a place in industrial playbooks thanks to shifts toward better solvency and flexibility in polymer and paint technologies. In my early career days, walking through factories and paint shops, I saw how the jump from simple alcohol-based solvents to complex glycols brought much-needed improvements to everything from print inks to flavorings. Tinkering with ethylene oxide and glycol transformations, chemists built up a family of compounds—each tweak opening up a new use. Diethylene Glycol Ethyl Ether Acetate, often abbreviated as DEGEA, stood out because manufacturers could dial in evaporation rates and boost compatibility with resins, leaving behind many headaches tied to older solvents.
This compound, often recognized for its clear and faintly sweet-smelling liquid form, bridges the gap between rough-and-ready solvents and those crafted for targeted performance. Instead of a one-note job, its make-up allows smooth interplay with both polar and non-polar systems. On a chemical order sheet, DEGEA appears under plenty of banners—sometimes as Ethoxyethoxyethyl Acetate or 2-(2-Ethoxyethoxy)ethyl acetate, depending on where you shop. It’s these detailed names that keep things straight in the warehouse and prevent the wrong drums from showing up at a customer’s doorstep. In my years in industrial coatings, the attention paid to labeling had real stakes; switching out just one letter in a name could mess up an entire production batch.
You could spot this solvent by its low viscosity and high boiling range—typically around 230°C. From one experiment to the next, this meant good stability, letting paint or lacquer dry without flash-rusting and keeping workers safer from uncontrolled vapor release. The surface tension is just high enough to give even coverage. Water solubility stands out as a practical blessing. Watching chemists blend it, I learned how that characteristic let the fluid stretch further in water-based applications—one less thing to source, more budget kept in place. The mild odor makes it less offensive than other industrial choices, which goes noticed in close-quartered workshops and manufacturing floors.
Technical data sheets give the nitty-gritty numbers: purity typically above 98%, specific gravity close to 1.0, and limited water fraction. Manufacturers standardize by setting strict thresholds for acidity and limiting residual starting materials like diethylene glycol or free acetic acid. Each container must be labeled with the United Nations code (UN number), bulk chemical name, concentration, and hazard pictograms. Running inventory audits during my stint as a compliance officer, one missed label meant a shutdown or docked pay—a real-world reason to keep specs visible and unambiguous.
Getting to the finished solvent involves reacting diethylene glycol monomethyl ether with acetic acid anhydride, all worked up under specific reaction temperatures and catalytic conditions. Unlike some rough-and-tumble organic syntheses, the process here doesn’t crank out clouds of byproducts. You track temperature, you meter your addition rates, you monitor pH swings. Batch records capture everything. In every plant I stepped into, engineers harped on electronic control as key to both quality and lower waste. Purification—often through distillation or liquid-liquid extraction—requires vigilance, and the team runs batch samples through gas chromatography to ensure consistent output.
Broad chemical compatibility sets DEGEA apart. The acetate group enables mild hydrolysis under acidic or basic conditions, handing formulators a solvent that adapts to many regimes without breaking down in unpredictable ways. One can attach or swap side groups for new variants, which brings fresh solubility or boiling points. Over a decade of visiting research labs, I’ve seen teams work overtime to adjust these features for tougher regulatory demands. Tinkering with the backbone chemistry, they chase ways to reduce toxicity or amp up biodegradability, sometimes adding pendant groups to shift solvent polarity.
Every distributor and supplier has their own pet names for this compound. Sometimes you’ll find it called “DGEEA,” or under trade names made up by multinationals aiming to lock in loyal buyers. In older documents, you might run into terms like “2-(2-Ethoxyethoxy)ethyl acetate,” “Ethylene glycol monoethyl ether acetate,” or “Acetic acid, 2-(2-ethoxyethoxy)ethyl ester.” Supply chain mix-ups and missed safety data stem not from chemistry mistakes, but from using the wrong synonym at the wrong time. Early in my regulatory work, I watched a shipment delayed three weeks just because the invoice and shipping label disagreed on the product name.
Worker safety requires clear protocols. DEGEA does not carry the same acute toxicity as some chlorinated solvents, but it comes with real risks. Direct skin and eye contact cause irritation. Prolonged inhalation or accidental swallowing in large amounts harms organs or disrupts the nervous system. The material safety data sheet—one form stapled above every workbench I ever visited—warns about keeping good ventilation, using nitrile gloves, and wearing splash goggles. Spill procedures focus on containment using sand or vermiculite, with prompt disposal in chemical waste drums. Fire safety rules recommend storing the solvent away from oxidizers and open flames, echoing lessons learned from too many warehouse accidents caused by cross-contamination or human error. Training new staff means walking them through not just data, but real examples of close calls or best-practice routines.
You’ll find this compound everywhere—in high-gloss automotive coatings, flexible plastics, printing inks, and sometimes even in specialty cleaning agents. In the paint store down the street, workers mix DEGEA daily for finishes that resist scratching and stand up to rough weather. Electronics manufacturers value its gentle solvency, allowing cleaning and solder flushing that leaves delicate circuits intact. Medical devices incorporate plasticized films that depend on this solvent for fine balance between stretch and toughness. In all those scenarios, consistency counts—it means less waste, fewer recalls, and more trust in the end product.
Labs continually test the limits of DEGEA, striving to trim the toxicity while keeping the industrial perks. Analytical chemists measure migration rates in packaging or test breakdown rates during incineration. There’s an uptick in projects targeting green chemistry—using enzymatic catalysis instead of heavy acids to generate the ester, or tweaking molecular structure for faster biodegradation. Teams adapt formulations to comply with new environmental codes and new toxicology findings. My own close-out report from an R&D project highlighted the shift to using plant-derived feedstocks to reduce carbon footprint, and those reports now influence boardroom decisions.
Studies between the 1980s and now paint a picture of moderate toxicity. Acute exposure can stress kidneys and liver, with high-dose animal tests showing developmental impacts that push regulators to set workplace exposure limits. Chronic low-level exposure worries researchers, especially in settings where air isn’t well-circulated. Regulatory agencies like the EPA and REACH keep pressing for updated research on metabolites, long-term bioaccumulation, and possible carcinogenicity. On-the-ground, this research translates to new emphasis on monitoring air quality in manufacturing and using personal protective equipment.
Industries look for replacements that check the same practical boxes but carry less health baggage. Biobased solvents are catching on—in pilot plants, teams swap petroleum-derived DEGEA for versions sourced from agricultural byproducts. That means rethinking supply chains and investing in new refining techniques. Europe pushes tighter regulation, squeezing demand for lower volatility and less persistent solvents. Even so, some sectors—specialty coatings, high-performance inks—show no sign of letting go, as the alternatives just can’t quite match the needed blend of performance and flexibility yet. The race to update product safety and tweak processing methods never slows down, led by the push to blend health, performance, and cost in a way that keeps both regulators and manufacturers satisfied.
No need to look far for the main stage of this chemical — walk through any construction site, automotive workshop, or home renovation store. Paints and coatings wouldn’t spread evenly or last long without a smart set of solvents behind the scenes. Diethylene Glycol Ethyl Ether Acetate (DEGEEA) steps into this role because it breaks down pigments and resins, making thick substances workable. Without it, heavy lacquer turns into an unwieldy goop, and industrial coatings start drying before the worker even puts down the brush. Car manufacturers, for example, rely on this solvent to keep paint jobs smooth and hardworking, especially as worldwide standards for finish and appearance keep rising.
A print shop with high-speed machines can’t use just any old ink. If ink dries too fast, the job jams up and heads clog. Dry too slowly, and sheets stick together and customers call with complaints. DEGEEA helps keep inks liquid enough to run through machinery at pace, so color settles perfectly on the paper. This plays out every morning with newspapers and packaging, where any hiccup costs money and time. While water-based systems become more popular, experienced print managers know there’s still a need for specialty solvents when quality truly counts.
Chip makers and electronics fabricators deal with incredibly sensitive processes, where a single fingerprint or stray fiber can spoil an entire batch. DEGEEA’s excellent dissolving skill works wonders in the gentle cleaning phases for printed circuit boards and microchips. For these manufacturers, it's like having a detergent that never leaves anything behind, so traces of flux, oil, or dust get erased before they cause expensive failures down the line. Technologies keep shrinking, and margin for error disappears with every generation — so the chemical recipe behind circuit cleaning hardly gets left to chance.
Think of industrial flooring, windshields, or even smartphone screens. These jobs use adhesives that must stay sticky for precise application and then harden for life-long strength. DEGEEA makes it possible to fine-tune that sweet spot between workability and full cure. Without a reliable solvent, workers either struggle with glue that hardens too fast, or they wait forever for a sticky mess to dry. As factories chase efficiency, every little edge on fast, reliable bonding means less downtime and more consistent quality.
Solvent use comes with a price — health risks, waste, and tricky disposal rules. Some factories look for greener alternatives, but swapping out effective chemicals is never just a paperwork swap. It involves retooling production lines, retesting quality, and often adding cost. Workers I’ve met in manufacturing value clean air and safety, so there's a steady push for better ventilation, personal protection, and stricter limits on how much is used at once. Researchers keep investigating substitutes, but until something matches DEGEEA’s balance of cost and performance, most industries still reach for it when old standbys fail.
Options for improvement don’t all sit in the chemistry book. Companies can adopt closed-loop systems that capture vapors, so less spills into the workplace. Investment in staff training pays off, too — seasoned workers spot leaks early and don’t take dangerous shortcuts. On the supplier side, transparency about what goes into blends helps buyers pick safer options and manage their risk. DEGEEA does some of the heavy lifting across manufacturing, but the conversation about smarter, safer alternatives and practices can’t rest on autopilot.
Diethylene Glycol Ethyl Ether Acetate—most people just call it DGEEA in the lab or paint shop. This chemical often pops up in coatings, inks, cleaners, and other industrial products. Many folks don’t realize how quickly the fumes can build up if you stash it in the wrong spot. Even in places with some airflow, vapors can collect, especially if windows often stay shut or the drum leaks.
I remember helping a friend clean up a minor spill in an art studio that used solvents like DGEEA. A forgotten, loosely capped jug had tipped and we all choked on the sharp, sweet smell before even spotting the problem. At that moment, ventilation didn’t seem like some distant guideline you can ignore. A big metal fan and opening more doors cleared the fumes, but no one wanted a repeat.
DGEEA catches fire easily. Even a spark from dragging a metal tool or switching a faulty light could set off problems if vapors have pooled. Safety manuals always push for flameproof storage rooms and cabinets for a reason. Flammable liquid cabinets with well-fitting doors keep things contained in case trouble starts. While the official storage temp range says “cool and dry,” money spent on air conditioning or dehumidifiers pays off by cutting risks. Any time you ignore heat and humidity, those risks climb.
Some assume you can sniff out danger, but DGEEA’s health hazards can show up without warning. Breathing too much in, especially over days, affects the nervous system and irritates eyes and lungs. Long sleeves and gloves do more than keep you clean—they shield against skin absorption, which can sneak up if you splash during mixing or pouring. I’ve seen people wash their hands in a hurry, only to find irritation or headaches hit later. Face shields and goggles seem like overkill until somebody forgets to blink away a droplet in their eye.
Moving large containers carries its own risks. Drums and bottles roll off carts a lot easier than you’d think, so using spill-proof carts, drum dollies, and secure lids matters as much as the gloves you wear. If a spill happens, don’t rush in with just a rag—roll out absorbent pads made for solvents and neutralize before sweeping up. Even small drips in the wrong spot can eat away at floor coatings, which ramps up slip dangers and makes clean-up longer and more expensive.
Mixing up your waste routine with improper chemicals can start slow reactions, building up heat or forming vapors. DGEEA containers should never mix with acids, strong bases, or oxidizers. Labeling every drum and tracking dates does more than keep auditors happy—it helps crews know what sits in storage and what needs disposal soon. I’ve seen teams cut down on emergencies by setting up color-coded labels and a quick checklist for weekly inspections.
Looking out for coworkers means more than shouting reminders. Organize regular short briefings so new staff actually see how to store and use DGEEA safely. Muscle memory builds from repeating good habits—wiping down lids, double-checking caps, and keeping clear old rags and materials from workbenches. Every step lowers the chance you’ll read about your own accident in tomorrow’s safety bulletin.
Everyone’s looking at labels these days, whether it’s food or something you find in a lab. Diethylene Glycol Ethyl Ether Acetate keeps things simple in that department. Its chemical formula is C10H20O4, and the CAS number is 112-15-2. That’s the unique fingerprint chemists around the world recognize. If you work in a coatings factory, a paint plant, or even in a university chemistry department, you’ve probably run across this formula on a barrel or a bottle. These codes aren’t just trivia; they keep folks safe and supply chains honest.
Mixing up chemicals on paper or in practice leads to disaster. Take it from someone who’s cleaned up a storage room after an “oops”—labels need to be clear and correct. People use this solvent in jobs where precision counts. Some see just a string of letters and numbers, but in real terms, that’s how researchers avoid blending the wrong solvent into industrial paint or electronic cleaning supplies. There’s a comforting certainty knowing each mixture comes with its own ID badge.
Chemical catalogues and safety sheets can look dull, but this particular compound turns up on a surprisingly wide range of sites. You spot C10H20O4 in factories working with automotive finishes, cleaning inks off high-speed printing presses, helping electronics manufacturers prep circuit boards. It helps paints spread smoothly instead of clumping and makes ink dry right where you want it. For anyone who’s ever painted a car, a sign, or even an apartment, the smooth flow comes thanks to solvents like this one.
My experience storing drums of Diethylene Glycol Ethyl Ether Acetate taught me respect for proper chemical handling. Gloves, goggles, and good ventilation save more than just clothing. Inhaling too much vapor, or landing a splash on your skin, brings on headaches and worse if you’re not prepared. The Material Safety Data Sheet—always labeled with that CAS 112-15-2—spells out those risks. Don’t ignore it. Safe storage and regular training keep people healthy and businesses running without surprises.
Sourcing chemicals can become a wild goose chase. Unscrupulous suppliers sometimes sell blends or substitutes that only look similar on paper. That’s where the chemical formula and CAS number save the day. When I ordered C10H20O4 from a new distributor years ago, double-checking the CAS saved my team from receiving a lower grade product. Nobody wants to find out mid-project that their base solvent isn’t what was promised.
Nothing stops everyone from pushing for better labeling, clearer communication, and stricter enforcement. Digital inventory systems reduce errors and catch mismatched products early. Regular training helps folks on the floor recognize a formula and understand what it means for their job. Companies can work closely with trusted suppliers and always ask for transparent sourcing—no shortcuts. Every person along the way, from shipping clerk to lab tech, should feel empowered to double-check and ask questions about what they’re handling.
So much depends on something as simple as a string of letters and numbers. C10H20O4 and CAS 112-15-2 don’t just belong in chemical textbooks—they matter wherever safety and reliability count.
Most people won’t find a bottle of diethylene glycol ethyl ether acetate (DEGEEA) under their kitchen sink. It usually shows up in places like paint factories, printing ink plants, or certain cleaning and coating product warehouses. I once worked for a summer at an auto-body paint shop, and chemicals like this were common. In those environments, risk awareness can slide unless safety culture stays top priority.
DEGEEA is a clear liquid that evaporates more slowly than some other solvents. That property makes it useful, but it’s also a double-edged sword. Longer evaporation means more time for workers to breathe in the fumes if the ventilation doesn’t keep up. Studies from the CDC and the European Chemicals Agency flag this solvent for eye and skin irritation, even at low concentrations. It’s not the most acutely toxic thing in an industrial setting, but steady contact does add up.
Breathing high concentrations can lead to headaches, dizziness, and nausea. Over a long shift, especially if you’re not paying attention or the room is hot and stuffy, those little signs can slip by. Splash it on bare skin and you can wind up with dryness or rashes—a lot like what happens when exposed to paint thinner routinely.
Worry starts to rise because of diethylene glycol in the molecule—remember the tragic stories about DEG contamination in medicines overseas. On its own, DEGEEA won’t kill you after a single whiff, but repeated exposure deserves respect. Animal tests suggest liver and kidney effects after enough contact, and there’s evidence to suggest some reproductive risks if inhaled or taken in through the skin over many months. Occupational safety bodies regularly list it as a substance requiring training and careful handling for a reason.
To lower the risk, companies need to go beyond handing out a pair of thin gloves and a dust mask. Building-level exhaust fans or local fume extractors make a big difference. Centralized chemical storage and good labeling avoid mix-ups. Based on personal experience, enforcement slips when everyone gets comfortable. Designated chemical handling areas and visible emergency instructions help keep the message front-of-mind.
Using the right gloves—nitrile, not latex—blocks skin absorption. Lab coats and face shields should come out if there’s even a remote chance of splashing. Changing contaminated clothes immediately and having proper eyewash stations at arm’s reach can spare serious health problems. Workers benefit most from bite-size safety training, ideally with real stories or accident reports on hand, rather than just dry warnings in a binder.
Safer alternatives exist for some of DEGEEA’s uses, especially in paints and inks. Green chemistry is making headway—plant-based solvents or new water-based formulations can often do the same job with less hazard. Change takes time, but market demand for safer products grows every year, pressuring companies to reconsider older chemicals.
DEGEEA isn’t the stuff of chemical thrillers, but treating it casually is a mistake. Like many industrial solvents, its danger level sits in the day-to-day habits—good ventilation, strong PPE culture, and education about long-term risks make the biggest impact. The cost of skipping those steps usually shows up years down the line, in medical bills or lost workdays nobody saw coming.
Industrial chemicals sometimes sound intimidating, but their safe use often just comes down to respecting a few basic rules. Diethylene Glycol Ethyl Ether Acetate, or DEGEA for short, demands much of the same level-headed treatment as many other solvents. The stakes feel real: misuse can compromise both workplace safety and product quality, and few people want to find out what happens after vapor leaks or old, degraded chemicals get drawn into a sensitive process. I remember seeing the inside of a cramped, stuffy backroom with mismatched labels and rusty cans—a place that practically dared chemicals to lose effectiveness or spill. That memory still shapes the way I look at storage practices.
DEGEA doesn’t last forever. Two years from the date of manufacture is a figure that most suppliers quote, assuming the container stays sealed and handled sensibly. Many labs and production teams rarely push it past that point, since chemical breakdown sneaks in as air and moisture work their way past seals. Even in less critical work, using old stock turns into a gamble, with each drum or pail an unknown. Discoloration or odd smells act as early red flags, but not every change is visible. Product performance can slip before anyone notices. Keeping a tight log—marking date received, date opened—lets everyone know where they stand. Even experienced teams slip up without clear records and honest audit trails.
Heat and sunlight remain the sworn enemies of DEGEA. My own time in cramped warehouse corners on hot afternoons taught just how fast the temperature can swing. Anything above 30°C adds risk. Too much warmth triggers reactions that speed up aging. Ultraviolet light can nudge the process along, degrading the acetate faster. Proper practice means picking a spot out of direct light, where temperature changes stay mild throughout the day. Shelving at waist or chest height works better than damp, cold concrete near the floor. Secure, upright placement keeps any leaks simple to spot, and protects workers from spills that could turn nasty in close quarters.
Moisture seems harmless—a bit of humidity doesn’t feel like much. DEGEA tells a different story. Water sneaking into drums encourages unwanted chemical reactions. Corrosion, cloudiness, and changes in volatility all crop up more often in poorly sealed or leaky containers. There’s no substitute for a tight seal or a quick wipe down after every pour. For smaller facilities or field use, airtight glass bottles with solid seals beat cheap plastic every time, especially in humid climates where you can’t always spot leaks until it’s too late.
Neglect leads to waste. I’ve seen workshops throw out liters of solvent that turned thick or started smelling like vinegar—money lost, downtime that grates on everyone’s nerves. More dangerous, failing to catch vapor leaks or cross-contamination can spark health worries or even legal trouble if spills escape containment. Rooms fill with fumes fast, and that sharp, unfamiliar smell should prompt evacuation, not a shrug. Safety gear—gloves, goggles, fitted masks—sits on the front line, but proper storage drops risks before problems even start.
Stickers, logs and regular checks cost next to nothing, yet too many skip them under pressure from deadlines or plain old forgetfulness. Every team benefits from a reliable system: check seals, rotate old stock before new, and train every new hand in what’s normal and what’s not. Chemical suppliers share their best recommendations in every shipment, but real safety culture grows in the daily habits of people who work around these fluids. Simple routines, real respect for the rules, and a sense of ownership make the difference between a safe, reliable shop and one on the edge of a costly or dangerous mistake.
| Names | |
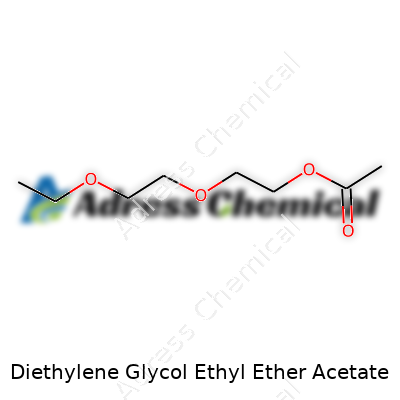
| Preferred IUPAC name | 2-(2-Ethoxyethoxy)ethyl acetate |
| Other names |
DEGEEA 2-(2-Ethoxyethoxy)ethyl acetate Ethylene glycol monoethyl ether acetate Diethylene glycol monoethyl ether acetate Acetic acid, 2-(2-ethoxyethoxy)ethyl ester DEGEE Acetate |
| Pronunciation | /daɪˈɛθiˌliːn ɡlaɪˈkɒl ˈiːθəl əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 112-15-2 |
| Beilstein Reference | 1105160 |
| ChEBI | CHEBI:89670 |
| ChEMBL | CHEMBL1351736 |
| ChemSpider | 84983 |
| DrugBank | DB14676 |
| ECHA InfoCard | 03b072c2-9568-44e6-bd3e-e4e5b6a6c0c7 |
| EC Number | Index No: 607-195-00-7 |
| Gmelin Reference | 68298 |
| KEGG | C19699 |
| MeSH | D017542 |
| PubChem CID | 12006 |
| RTECS number | KC8750000 |
| UNII | LL2P7U82CB |
| UN number | UN1171 |
| CompTox Dashboard (EPA) | DTXSID5043292 |
| Properties | |
| Chemical formula | C10H20O4 |
| Molar mass | 190.23 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild ester-like |
| Density | 0.997 g/cm³ |
| Solubility in water | miscible |
| log P | 0.64 |
| Vapor pressure | 0.025 mmHg (@ 20 °C) |
| Acidity (pKa) | pKa ≈ 15.5 |
| Basicity (pKb) | 8.2 |
| Magnetic susceptibility (χ) | -52.0e-6 cm³/mol |
| Refractive index (nD) | 1.419 |
| Viscosity | 1.7 mPa·s (25 °C) |
| Dipole moment | 4.76 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 402.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -663.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3790.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause drowsiness or dizziness. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H302 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378, P403+P235, P501 |
| Flash point | 77°C |
| Autoignition temperature | 225 °C |
| Explosive limits | 1.0% - 10.0% |
| Lethal dose or concentration | LD50 (oral, rat): 5,247 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5,500 mg/kg |
| NIOSH | KCA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 ppm (27 mg/m3) |
| Related compounds | |
| Related compounds |
Diethylene glycol ethyl ether Diethylene glycol methyl ether acetate Ethylene glycol ethyl ether acetate Propylene glycol methyl ether acetate Diethylene glycol monoethyl ether |