Chemical manufacturing keeps stretching boundaries, chasing both productivity and safer, smarter compounds. Diethylene Glycol Butyl Ether Acetate (DGBEA) doesn’t show up with the hype of newer green solvents, but its roots go back to a period when folks in labs leaned hard on glycol ethers for paint and coating breakthroughs. Years ago, manufacturers faced problems with drying times, film quality, and handling characteristics, and early explorations into new glycol ether acetates like DGBEA rewrote the playbook. Companies wanted less harsh evaporating agents for industrial and household paints, and scientists dug into glycol esters for flexibility and longevity. Over time, companies tweaked and experimented—not chasing novelty for its own sake, but out of the simple reality that factory floors needed less toxic, more effective, and stable compounds. DGBEA isn’t a household name, but its history traces every bit of this hard, practical graft in chemical development.
DGBEA crops up most in paint thinners, industrial cleaners, and as a base for specialty coatings. The colorless, nearly odorless liquid hosts a familiar lineage, related to its ether and acetate cousins, but it's crafted for solvent strength and better compatibility in demanding blends. Most folks working with paints or inks brush shoulders with compounds like DGBEA, rarely naming it, but appreciating that products glide on surfaces cleaner and level more predictably. The paint doesn't dry in the can too soon. Factories trust this liquid because it bridges the gap between strong enough for professional use, yet not so harsh that it kicks up a fuss with safety managers or workers. This broad utility puts it right at the line where performance and reliability meet daily industrial needs.
DGBEA has a molecular formula of C12H22O5, tipping the scale near 246.3 grams per mole. Measuring density tells you a lot in applications—it hovers around 0.98 g/cm³, thicker than water but easy to blend. If you’ve had to store or ship chemicals, you watch for its flash point, which sits at roughly 96°C, putting it safely away from the unpredictable fumes of lighter solvents. Water solubility lands in the low to moderate range, opening up blending options without worrying about excess water interference. Most impressive, it holds a stable boiling point above 250°C, rare for common glycol ethers, and this keeps drum storage and plant handling straightforward. A long liquid phase window means coatings, cleaners, and specialty inks don’t have to play guessing games on evaporation or mixing problems during a shift.
Drums or totes marked for DGBEA always list the CAS number 124-17-4, a safeguard for both plant technicians and regulatory compliance. Purity for industrial-grade stock is tracked closely, usually topping out above 99.5%, with moisture content and acidity below tight thresholds. Specs sheets show color as “clear to pale yellow”, but many users gamble with unbranded material and run into surprise residues or off-odors. This stresses why consistent supplier checks matter—companies keep quick tests on hand and insist on origin certification, protecting their brands against costly recalls or batch failures. On labels, hazard pictograms highlight mild skin and eye irritation, but careful shelf storage and good air circulation settle most day-to-day worries.
The production process usually starts by reacting diethylene glycol monobutyl ether with acetic acid, running under acid catalysis. In any plant I’ve seen, the reaction kicks up a bit of heat, and product separation needs careful distillation: a balance of skill and precise control, not just rote following of protocols. Removing byproducts, usually water and some excess acids, takes undistracted monitoring—if shortcuts creep in, end-use coatings or solvents take the hit. Efficient facilities get strong yields and high purity, while slipshod operators face nastier impurities and disposal costs. In my own career, teams would compare lots week by week, always striving to polish the yields one fraction higher, understanding that clean process saves both time and nerves down the line.
Handling DGBEA centers around its ester linkage—this functional group doesn’t just sit still in a beaker. Alkaline environments threaten to hydrolyze it back into glycol ether and acetic acid, and high heat can break it down in unpredictable ways. Add it to coatings with strong bases, and you get clouding or drop in solvent strength. Specialty applications sometimes exploit this reactivity, flipping DGBEA into secondary derivatives for adhesives or cleaners. Modifications don’t just happen in R&D labs; on the ground, plant technicians have to watch blend ratios and storage temp, because residual acidity or unintended breakdown can mess with batch consistency. Workers who take shortcuts risking exposure to caustic, learn fast that vigilance beats complacency.
DGBEA often hides behind names like “Butyl Carbitol Acetate”, “Diethylene Glycol mono-n-Butyl Ether Acetate”, or the simple “Carbitol Acetate”. Chemical traders may even market by in-house codes or legacy trade names, confusing newer staff or those used to European naming conventions. I’ve seen shipments returned or stuck in customs just because labels didn’t match expected import registry names, pointing to the big headache inconsistent product nomenclature triggers. Responsible companies stress familiarity with all synonyms—and consistently train workers before letting any new material through the door. This attention to detail stops costly mistakes before they pick up momentum.
DGBEA sits in the middle ground of practical solvents: exposure through skin or respiratory intake stings, but it doesn’t send teams scrambling like more volatile ethers or strong acids. Safety guidelines still expect gloves, splash goggles, and mechanical ventilation, because repeated contact causes dermatitis or eye irritation. Any facility using bulk solvents keeps spill absorbents and flammability charts handy, and seasoned handlers double-check temperature controls to steer clear of fire risks. Long-term exposure tracking keeps the health team in the loop; some staff over the years get careless, and end up battling unnecessary chronic hand irritation or mild respiratory trouble. True safety doesn’t wait for an incident, and the best crews drill basic protocol until it’s routine, not just mandatory paperwork.
Paints and coatings stand as the core market for DGBEA, especially in industrial zones where high-performance finishes or textured films are standard. Printing inks gain smoother flow and extended drying windows. In electronics or automotive plants, the chemical supports cleaner application of protective films, reducing rework or surface defects. Cleaners and degreasers benefit from DGBEA’s ability to dissolve greasy residues without the harsh edge of old-school solvents—using it for floor stripping or machinery wipes limits harm to both substrate and operator. Flexible enough for textile dyes and inkjet printing, the compound proves its worth in both heavy industry and precision equipment. End uses might look diverse, but they all rely on chemical balance, managed evaporation, and a history of reliable results. Having watched production lines stop due to unpredictable drying or streaking, I’ve gained new respect for compounds like DGBEA that quietly keep these systems humming along.
Lab teams spend their time not chasing novelty but refining how DGBEA-based blends outperform old solvent systems, especially under new regulatory or environmental rules. Practical improvements come from side-by-side field tests, not just theoretical gains—measuring film hardness, surface tack, or cleaning efficiency right on the shop floor. Collaborations with academia also feed better understanding, such as studies on how tiny tweaks to glycol chain length can stretch or narrow drying rates. Older formulations get a second look every time new workplace exposure guidelines come out, forcing upgrades or reformulations to stay in the market. My experience watching these tweaks emerge, batch by batch, pointed out that innovation isn’t always dramatic; it’s often one small operational improvement at a time, stacking up until an entire facility changes over quietly in the background.
Toxicological review highlights that DGBEA isn’t without risk. Acute exposure in animal studies signals mild to moderate toxicity, though far below many classic solvent hazards. Chronic exposure—long days, unventilated rooms, or regular skin spills—promotes low-level irritation but not the organ damage seen with heavier glycols or older acetates. Studies track inhalation threshold values and screen for possible reproductive or developmental side effects, yet most major findings flag manageable risks with basic PPE and modern handling standards. Companies invest in monitoring protocols, sending batch samples for review, and health data for regular audits. I’ve seen tight-knit operations drop incident rates through simple routine upgrades: better gloves, cleaner storage rooms, and personal air monitoring, long after academic reports fade from day-to-day memory.
Stricter environmental laws and pressure to drop volatile organic compounds force every chemical player to rethink old standards. DGBEA lines up as a transitional solvent—cleaner than old petrochemical mixes, not yet phased out by emerging green alternatives. Markets shift, and industrial chemists eye bio-derived or less toxic ethers for next-generation coatings. Still, replacements need performance that stands up to shop floor mess and long-term reliability, so DGBEA keeps its shelf space for now. Some manufacturers experiment with partial blends or water-assisted systems, cutting dependency bit by bit, but full switchovers never happen overnight. This slow evolution brings both uncertainty and opportunity: operations willing to invest in targeted R&D or risk smarter new blends might grab future market lead, but plenty of practical operators will stick with DGBEA as long as regulators allow, trusting a known performer over unproven change.
In my time working on a few painting projects—sometimes in too-small apartments—solvents always came up in conversation. Diethylene glycol butyl ether acetate, often shortened to DGBA, stands out in the paint and coatings world. This isn’t the sort of chemical you’ll find in school art class. Instead, it’s on the ingredient list for automotive finishes, industrial lacquers, and high-end architectural paints. The reason is straightforward: DGBA doesn’t flash off the surface quickly. Paint has a chance to smooth out, which cuts down on streak marks and brush strokes. Nobody wants a finish that cracks or sags. DGBA’s slow evaporation means a painter or sprayer can work longer with each batch, reaching a smoother, cleaner surface.
Factories and workshops can’t get by with soap and water. Grease, oil, stubborn residues—these build up fast. Here, DGBA gets appreciated as a solvent that takes on challenging grime without reducing plastics, metals, or sensitive surfaces to a mess. Walking through an auto shop, you’ll spot mechanics using cleaner fluids for everything from brake dust to dried oil stains. Industrial users need solvents that break down junk but don’t wreck tools or parts. DGBA can get added to commercial degreasing formulas for that reason—effective cleaning, less surface damage, and not as intense a smell as some alternatives.
Print shops producing magazines, snack wrappers, or glossy cards turn to special solvents for their ink blends. DGBA earned a place here thanks to how it controls drying times and keeps inks flowing evenly. With demand for sharper graphics and thinner films, traditional solvents fall short. Back when I used to help design packaging, any ink that dried too soon or stayed sticky risked a whole batch being tossed out. DGBA helps balance the working time, so high-speed presses don’t get clogged and prints aren’t smeared or misaligned. In flexible packaging—think chip bags or juice pouch labels—the solvent must avoid wrinkling delicate layers. DGBA’s performance fits the bill.
Few people realize how solvent blends shape the world of electronics. Making circuit boards isn’t just about etching lines; it’s also about cleaning residues and prepping surfaces for precision soldering. DGBA finds its role in electronics cleaning, where delicate parts can’t take harsh scrubbing or aggressive chemicals. A tiny leftover smudge can mess up an entire device. By using a solvent like this, tech manufacturers sidestep bigger problems down the line, from faulty connections to short circuits.
It’s impossible to ignore that solvents, including DGBA, pose their own risks. Breathing fumes isn’t healthy; skin contact needs to be avoided. On some shop floors, poor ventilation and weak safety rules turn a useful solvent into a hazard. Green chemistry has started to push companies towards safer, less toxic solvent systems. But change comes slowly, especially where DGBA does the job better than newer alternatives. The move forward relies on tighter handling rules, better ventilation, and real investment in alternatives that match both safety and performance.
Every chemical comes with its own identity code, a CAS number, and for diethylene glycol butyl ether acetate, that number is 124-17-4. This isn’t just a string of digits for warehouse shelves. I once worked in a small auto-detailing shop, and our manager drilled into us the importance of checking labels and numbers, mainly to avoid the kind of mix-ups that leave paint jobs ruined or workers exposed. A slip—using a near-identical solvent without double-checking that number—could leave someone nauseous or cost the shop hundreds on a ruined job. The CAS number is the backbone for safety data sheets, procurement paperwork, and shipping manifests.
Chemical names tend to blur together or come with half a dozen aliases. The CAS number, though, offers clarity where letters fail. In my college chemistry lab, it didn’t take long to appreciate why filing cabinets and bottles both wore those numbers like a badge. With diethylene glycol butyl ether acetate, I’ve watched suppliers refer to it as DEGBEA, butyl carbitol acetate, and sometimes names I’d never heard. There’s no guessing with CAS 124-17-4—it cuts through confusion fast.
Production managers and warehouse staff live by these codes. Everyone in the chain needs to know exactly what’s in those drums. I’ve seen one letter swapped on an order form for a small manufacturer, which led to delivery of an entirely incompatible solvent. That single error could have set back the production schedule for weeks. With the right CAS, businesses keep their processes tight and regulated, with fewer headaches or dangerous slip-ups.
Working with chemicals like diethylene glycol butyl ether acetate doesn’t just mean pouring liquids or mixing solutions; it means navigating regulations and liability, too. Organizations like the EPA and OSHA track chemicals using CAS numbers, so compliance teams refer to CAS 124-17-4 to check reporting obligations or exposure limits. Health workers treating workplace accidents need that code to get the right protocols in place. Insurance adjusters, government auditors, and environmental consultants rely on these same digits to do their jobs quickly and accurately.
Clear labeling stands out as one simple fix. I’ve worked in places where bottles only had names scratched on with a marker—those were the shops most likely to scramble for an MSDS in the middle of an accident. Printing the CAS number boldly on every storage container, order sheet, and manifest removes guesswork. Digital tracking systems should make CAS entry mandatory, especially for orders or inventories. For teams working across languages or shifts, that code unifies everyone in a way that long chemical names never could.
Teaching new hires about the meaning of the CAS number does more than check a box on safety orientation. It can keep people healthy, orders accurate, and projects on track. The difference between workplace chaos and calm often starts with three sets of numbers—like 124-17-4—staring back from a well-placed label. Sometimes, the simplest identifiers carry the biggest impact.
At first glance, Diethylene Glycol Butyl Ether Acetate may sound like chemistry class jargon, but most of us have brushed up against it in common products. This compound shows up in paints, coatings, inks, and cleaners. Its job is to dissolve or carry other chemicals so things spread evenly and dry with minimal marks. Factories like using it because it makes the process smooth and reliable—even under pressure.
Daily exposure to many industrial chemicals passes with zero side effects, but not every case works out that way. Touching or breathing in Diethylene Glycol Butyl Ether Acetate can irritate the eyes, nose, and throat. Some workers report headaches or feeling dizzy after long shifts in rooms where the air can’t circulate well. This isn’t just theory. Studies have shown high levels may impact the nervous system and even long-term kidney function if contact continues without proper gear.
Once, in an old paint shop I worked at, we had a lone exhaust fan—and we learned the hard way that wasn’t enough. After a day smoothing out baked-on finishes, my coworker and I felt light-headed, hands tingling. That metallic taste in the mouth wasn’t lunch; it came from the vapors hanging around us all day. Only later did we figure out that air circulation mattered as much as gloves and goggles.
Factories and workshops sometimes let chemicals seep outside their doors. Diethylene Glycol Butyl Ether Acetate stands out because it doesn’t hang around forever—it breaks down when exposed to air and sunlight. Even so, water runoff causes the biggest headaches. Spills or careless waste can end up in rivers or groundwater. Once in water, it breaks down, but fish and insects face stress along the way.
A spill in a small stream can set off a chain reaction. Fish get sluggish or die off in spots. These aren’t always massive events, but every new contaminant adds to a slow build-up in the local ecosystem. Back in my hometown, our river once turned oddly foamy. The mystery cleared up after residents traced it back to a chemical plant upstream—shortcuts in waste disposal added toxins like this solvent to the mix.
Some solutions feel almost too simple: better ventilation and protective clothing. Employers who focus on these strategies notice that sick days drop and jobs get done with fewer mistakes. Proper storage cuts accidental spills and limits air leaks. Labeling containers and setting up regular training sessions helps everyone stay sharp.
On the bigger scale, stricter waste controls by businesses keep waterways safer. Cities that invest in decent sewage treatment stop dangerous levels of solvents from slipping into community water supplies. Laws matter, but constant reminders and a sense of responsibility mean even more in day-to-day routines.
Ignoring the risks tied to Diethylene Glycol Butyl Ether Acetate rarely turns out well for either people or their surroundings. Choices at work, at factories, and beyond show up in real outcomes: fewer headaches on the shop floor, cleaner water in backyards, and local wildlife that keeps its balance. Small steps—wearing a mask, checking a label, calling out poor waste habits—add up, and everyone gets the payoff.
Anyone who’s worked around industrial chemicals knows that good habits in storage and handling keep people safe and businesses running. Diethylene Glycol Butyl Ether Acetate isn’t something most people bump into at the grocery store, but paint shops and chemical plants see it daily. I remember my first time uncapping a drum; the smell alone said this wasn’t something to take lightly.
Chemicals like this act like a bad roommate if left unchecked—sticky, persistent, and quick to get out of hand. I’ve seen shops lose thousands when solvents got contaminated. Poor storage can mean ruined stock, employee sick days, or far worse. According to the National Fire Protection Association, mishandled solvents account for dozens of industrial incidents every year. Let’s talk about some ways to keep your operation on the right side of those stats.
This liquid behaves best at room temperature. A space that runs too hot speeds up evaporation, which kicks volatile fumes into the air and nudges the risk of fire up. Cold storage tanks won’t benefit you much, but an overheated shed invites trouble. I once saw storage in a tin shed in July—the waste and cleanup after a leaky container popped in the heat left a lasting impression. A well-lit vent fan and a thermometer can save big headaches by keeping things steady.
Fresh barrels come tightly sealed and labeled. Out in the wild, though, that seal breaks when folks take shortcuts. Always stick with the original drum or approved secondary container. Polyethylene or steel with good chemical resistance stands up better, while makeshift buckets and old soda bottles belong nowhere near this stuff. Drums should always get capped after each use, and dating them helps with rotation and inventory.
It sounds basic, but clear labeling prevents so many mix-ups. Permanent markers fade, so printed resistant labels hold up longer. I’ve seen the old tape-and-marker trick fail after a humid week. Keeping incompatible materials apart—like oxidizers or strong acids—could mean the difference between a normal Monday and a hazmat scramble. Make spaces with clear floor markings and signs. Good organization beats memory every time.
Full goggles, gloves, and work clothes block splashes. Even pros get too comfortable sometimes, and that ends up in the first aid station. Dermal exposure causes irritation, so avoid open-toed shoes or bare skin nearby. Lift with steady hands, and get a cart for heavy loads. Spills won’t wait for you to find that one absorbent pad, so keep cleanup kits accessible and training fresh for everyone on the floor.
This solvent isn’t the most flammable one on the shelf, but fumes still bite back with a spark. Class B fire extinguishers belong within reach, and ignition sources should stay far from storage. OSHA recommends periodic checks for leaks and corrosion—clamp down on puddles as soon as they turn up, and dispose of rags and pads used during cleaning according to local rules. Relying on wishful thinking beats getting blindsided by an inspection or accident.
Daily walk-throughs and checklists keep things running smoothly. I learned early to check lids, look for bulges in barrels, and note the smallest drips or sticky spots. Training isn’t just for new hires; regular refreshers keep experienced folks from cutting corners. Spills, evaporated drums, and ruined supplies shrink with steady attention. Turning chem safety into a daily habit keeps you ahead of the worst-case scenarios.
Anyone who’s worked with specialty chemicals knows purity isn’t just a technical detail—it shapes the end results. Diethylene Glycol Butyl Ether Acetate (let’s call it DGBEA) lands on the desks of chemists, paint producers, and coatings experts all the time. They care about purity because it feeds right into product safety, reliability, and performance; nobody wants to troubleshoot mysterious failures in a cured film or see coating defects blamed on a trace contaminant.
Most manufacturers ship out DGBEA at a purity of 98% or higher. The sweet spot is around 99%, measured by gas chromatography. Having handled specification sheets and purchased batches for lab work myself, I’ve noticed the line is drawn there—not out of tradition, but because impurities start causing trouble above that level. Residuals like diethylene glycol, butyl acetate, water, or colorants can spell headaches down the road.
Take water for instance. Most specs will cap moisture content at 0.1% max. Water in the solvent creates haze in coatings and slows drying, both real problems for anyone hoping to meet customer demands. Another target: acid value. Manufacturers set an upper limit—often around 0.05 mg KOH/g—to keep corrosion at bay and reduce the risk of unwanted side reactions.
Sitting on the other side of formulation tables, it’s clear—skimping on solvent purity invites frustration. In paints, traces of lower molecular weight alcohols throw off viscosity, affect open time, and push the application window past what the technical data sheet promises. I’ve seen coatings with microbubbles and craters because lower-purity solvent carried over unseen trouble. In cleaning products, off-spec DGBEA hits odor and stability, undercutting the very reasons people buy the stuff.
For buyers, getting the right DGBEA isn’t about picking the first supplier found on a directory. It pays to look at COAs (Certificates of Analysis), request independent testing, and pin down details on heavy metals, aromatic hydrocarbons, and residue on evaporation. Handling contracts for contract manufacturers, I’ve found a little paperwork upfront saves far more headaches than haggling over failed batches. Purity targets are a stake in the ground, and meeting them keeps production on track.
Industry groups like ASTM or ISO don’t always have a published grade for every solvent, but best practice has grown from experience. Producers get feedback from end users—paint defects, out-of-spec performance—and ramp up purification accordingly. Technologies like molecular distillation and adsorptive stripping help push impurity levels lower each year.
If things go wrong—say an uptick in off-odors or equipment fouling—auditing upstream sources for process contaminants brings real answers. Batch tracking catches shifting specs before a small problem starts eating margins or losing customers. In tough markets, suppliers leaning into robust purification and detailed batch tracking stand out for all the right reasons.
The line on purity for Diethylene Glycol Butyl Ether Acetate sits where chemistry, process safety, and end-product quality all meet. Getting that extra percentage point isn’t just a check mark—it’s how users maintain trust in finished goods, hold onto clients, and keep warranties on solid ground. Choosing high-purity sources isn’t a luxury—it’s insurance against avoidable problems and an edge in a field where details matter.
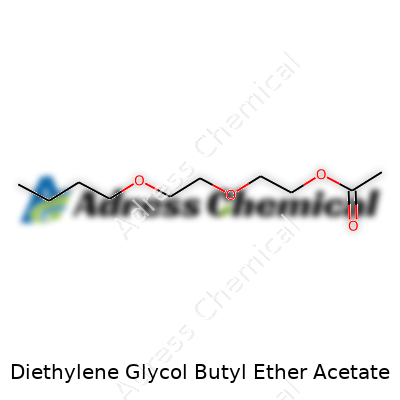
| Names | |
| Preferred IUPAC name | 2-(2-butoxyethoxy)ethyl acetate |
| Other names |
Butyl Carbitol Acetate 2-(2-Butoxyethoxy)ethyl acetate DEGBEA Butyl diglycol acetate Diethylene glycol monobutyl ether acetate |
| Pronunciation | /daɪˈɛθiːliːn ɡlaɪˈkɒl ˈbjuːtɪl ˈiːθər əˈsiːteɪt/ |
| Identifiers | |
| CAS Number | 124-17-4 |
| 3D model (JSmol) | `3d:JSmol\|CCOCCOCCOC(=O)C` |
| Beilstein Reference | 1722116 |
| ChEBI | CHEBI:83378 |
| ChEMBL | CHEMBL1853722 |
| ChemSpider | 157354 |
| DrugBank | DB13270 |
| ECHA InfoCard | InfoCard: 03-2119941958-35-0000 |
| EC Number | 203-933-3 |
| Gmelin Reference | 94744 |
| KEGG | C19699 |
| MeSH | D002948 |
| PubChem CID | 82997 |
| RTECS number | KO3150000 |
| UNII | F84HGL3558 |
| UN number | UN number: "UN3272 |
| CompTox Dashboard (EPA) | DTXSID5020703 |
| Properties | |
| Chemical formula | C12H24O5 |
| Molar mass | 246.32 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Mild ester-like |
| Density | 0.980 g/cm3 at 20°C |
| Solubility in water | Miscible |
| log P | 1.04 |
| Vapor pressure | 0.05 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 15.5 |
| Basicity (pKb) | 15.38 |
| Magnetic susceptibility (χ) | -66.5e-6 cm³/mol |
| Refractive index (nD) | 1.421 |
| Viscosity | 1.9 mPa·s (at 25°C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -830.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5101.3 kJ/mol |
| Pharmacology | |
| ATC code | NO ATC CODE |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| Flash point | 77°C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD50 (oral, rat): 6500 mg/kg |
| LD50 (median dose) | 2400 mg/kg (rat, oral) |
| NIOSH | N0204 |
| PEL (Permissible) | No OSHA PEL established |
| REL (Recommended) | REL (Recommended Exposure Limit) of Diethylene Glycol Butyl Ether Acetate: 10 ppm |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Diethylene Glycol Monoethyl Ether Acetate Diethylene Glycol Monoethyl Ether Diethylene Glycol Monobutyl Ether Diethylene Glycol Ethylene Glycol Monoethyl Ether Acetate Ethylene Glycol Butyl Acetate |