Butyl propionate’s journey runs parallel to the long fascination with esters and organic solvents. Chemists in the early 20th century didn’t overlook this compound as they explored the potential of esters for everything from fragrance synthesis to adhesives. The commercial interest in butyl propionate broadened as the coating industry expanded in the 1950s and 60s, when manufacturers prioritized both drying efficiency and pleasant odor. Compared to heavy, cloying solvents, butyl propionate offered a smoother profile that caught the attention of formulators trying to improve user experience and technical performance. Now, with regulations tightening and the push for less toxic alternatives, the background of butyl propionate matters more than ever, especially for those tracking how solvents moved from crude mixtures to sophisticated, highly characterized materials.
At its core, butyl propionate sits in that group of substances called esters, born from a union between butanol and propionic acid. This clear, mildly fruity liquid flows into everything from paints to inks and coatings because it dissolves many resins that others struggle with. Suppliers sell several grades tailored for high-performance applications, signaling how demand has shaped production standards. Often, laboratories turn to it as a benchmark for volatility and odor in solvent studies since its profile serves as a straightforward reference in this crowded market of esters and ethers.
Butyl propionate doesn’t hide much when poured onto a scale or sniffed at the bench. Boiling point settles near 146-149°C, and you get a liquid that isn’t viscous or sticky. That fruity smell isn’t just a curiosity—it impacts ventilation requirements and signals evaporation rates in solvent blends. Hydrophobic backbone, moderate polarity, and good solvating power mean it often appears in technical discussions as an alternative to more aggressive or hazardous solvents. Flammability remains a concern, as its flash point hangs near 36°C, leading handlers to treat it like any flammable organic with easy ignition. Water solubility stays low, less than 1%, so it lingers in organic fractions longer than you might expect in formulations.
Walk into any supply room and pull a drum labeled “butyl propionate”—you’ll see CAS number 590-01-2, a UN number 1276, and all the trappings of international chemical regulation. Purity often tops 98%, yet tighter applications in electronics or pharmaceuticals sometimes call for further purification, with GC and NMR confirmation. Labeling standards demand hazard pictograms for flammability as well as clear handling instructions. These standards don’t feel arbitrary; they respond to legitimate concerns in storage and transport, especially where solvents bump up against temperature swings or mixed loads. Documentation, from Safety Data Sheets to packaging labels, shapes the way people interact with the chemical every step from the loading dock to the lab bench.
Commercially, making butyl propionate means esterifying propionic acid with n-butanol, in the presence of a strong acid catalyst like sulfuric acid. Under reflux, water escapes as a byproduct, and technicians often employ Dean-Stark apparatus or dryers to squeeze out every last bit for maximum yield. The drive for purity doesn’t stop at synthesis—producers wash, neutralize, and distill the crude ester to chase out side-products and leave a colorless, stable compound behind. In practice, quality hinges on keeping water out and acid neutral during workup, because even slight contamination can throw off subsequent applications in sensitive coatings or inks. Large-scale plants rely on stainless steel and closed systems to maintain consistency, keep emissions low, and protect workers from fumes.
Butyl propionate’s structure tells a story of both resilience and reactivity. The ester group sits exposed when strong acids or bases show up, making hydrolysis a real risk in alkaline or acidic environments. Any attempt to transesterify will meet little pushback, so swapping out the alcohol moiety for a specialty variant works surprisingly well for manufacturers tweaking solvent properties. On oxidation, expect cleavage; harsh agents fragment the molecule. As a result, producers rarely blend it with strong oxidizers or caustic bases unless carefully controlled. Chemical engineers eye its backbone for custom modifications, especially when aiming for tailored evaporation rates or tuning polarity to dissolve trickier resins.
Look through catalogs, and you’ll find butyl propionate masquerading as butyl propanoate, n-butyl propionate, or even under old trade names tied to a specific supplier. Some paint and ink producers slap a proprietary touch on the naming, complicating procurement for buyers dealing with multiple suppliers. Despite the aliases, regulatory filings and customs declarations usually stick to the IUPAC or CAS conventions, which ties all these threads together for those mapping supply chains or reviewing archival material.
Anyone who’s spent time around organic solvents knows that flammability, inhalation, and skin contact always top the list of safety worries. For butyl propionate, proper ventilation isn’t optional—it bends toward easy vaporization. Workers handle it with chemical-resistant gloves and splash goggles, especially for drum-to-tank transfers, because the potential for splashes and inhalation spikes at scale. OSHA and similar bodies anchor exposure limits to short-term vapor concentrations, forcing facilities to adopt monitoring strategies and exhaust systems. Fire risk means that storage drums always stay in segregated areas, tagged and regularly inspected, often under foam-sprinkler systems in high-traffic sites. Protocols furnish spill kits and insist on regular drills, drawing lessons from both past incidents and regulatory updates.
Butyl propionate pops up across a landscape of industries. Paint-makers lean on it for rapid drying without pushing up odor—everyone from industrial metal coaters to furniture makers benefit. Printers see value in its ability to carry pigment and spread evenly on a range of substrates. The fragrance sector taps its sweet, fruit-like aroma, folding it into flavors and scents even as regulators ask for clear labeling in finished products. Some pharmaceutical formulations use it as a medium for poorly soluble compounds, but strict standards screen for residue and potential interference. Research labs answer questions about its solvent power for custom polymers, while specialty adhesive producers count on its balance of drying speed and solvency for both assembly lines and craft products. Even as environmental concerns grow, designers often find it hard to replicate the balance butyl propionate brings without a stack of alternative solvents and laborious testing.
New uses for butyl propionate continue to emerge as the pressure for safer, greener solutions rises. Scientists investigate biodegradability in both lab and environmental samples, hoping to match performance with a lighter ecological footprint. Technical groups at coating conferences highlight work around replacing higher VOC solvents with butyl propionate in both water-based and hybrid systems. Efforts stretch into optimizing synthesis, chasing enzyme catalysis or low-waste processes to post greener credentials. Analytical chemists push method development for trace detection in food and beverage contexts, responding to the compound’s use in flavorings and the rising consumer awareness of additive contents. The biggest shift comes from collaborative academic–industry teams pushing the boundaries of what these esters can do, ensuring yesterday’s workhorse chemicals don’t lag behind modern regulatory or technical demands.
Toxicologists haven’t ignored butyl propionate simply because it smells pleasant or works well; instead, they’ve run tests for everything from acute oral and dermal exposure to vapor inhalation. Animal studies show low to moderate toxicity at high doses, most symptoms showing up at levels far above daily industrial exposure. Irritation to eyes and respiratory passages gets more attention than systemic toxicity, fueling the use of protective equipment and closed transfers in production facilities. Chronic exposure data still brings some gaps, so safety officers encourage conservative controls in absence of clear long-term data. Environmental assays show the compound biodegrades relatively fast in soil and water, reducing persistent risk, but its volatility demands care near waterways and storm drains to prevent airborne releases from ending up where regulators most worry.
The future for butyl propionate looks a bit like a balancing act between performance, safety, and sustainability. Some analysts predict more blending with biogenic esters and a drift toward lower-VOC products, yet existing infrastructure and cost factors create inertia against sudden changes. Advances in green chemistry might usher in ways to produce or modify butyl propionate with fewer emissions and less energy, making it more palatable for both manufacturers and regulators. Research into recycling and recovery techniques at the facility scale shows promise, where users could capture and reuse evaporated solvent in closed-loop systems. Stakeholders from paint formulators to lab suppliers watch these trends closely, knowing that materials like butyl propionate will probably stick around, though perhaps transformed by shifts in how we think about solvents, safety, and the environment.
Butyl propionate pops up in more places than most people realize, but the solvent’s main gig is in paints, varnishes, and coatings. Most folks have probably caught a whiff of that sweet—and kind of fruity—note coming off fresh paint. Part of the credit goes to butyl propionate. Manufacturers prize this stuff for its ability to thin paint, helping pigments spread smoothly and stick better. It helps paint glide across walls without thick lumps or streaks.
People working in furniture finishing or automotive shops use products with this solvent because it helps create a glossy, even coat without constant re-dipping or sanding. Nobody wants to wait forever for something to dry, either, and butyl propionate makes sure drying doesn’t take all day. While its fumes have that almost pleasant odor, proper ventilation is always a must—prolonged exposure can be irritating or worse.
Printers and packaging plants have leaned on butyl propionate for decades, especially in inks. Printers need fast-drying, sharp images that don’t smudge. Inks with this solvent dry tight and bond to slick paper or film, a winning recipe for magazines, food wrappers, and all the marketing flyers you find in your mailbox.
Looking into household products, there’s a chance butyl propionate is hiding in cleaning supplies, waxes, or polishes. The solvent chops through oily residues, breaks down sticky messes, and then evaporates, leaving things looking clean instead of streaky. Even fragrance makers have taken advantage of butyl propionate’s light scent and fast evaporation to dissolve and deliver scents in everything from body sprays to room fresheners.
People can get used to a fresh paint smell or a sharp-clean room, but using chemicals like butyl propionate always means balancing usefulness with health. For those working around it every day, masks and good airflow in the workspace matter. Over time, strong solvents are known to cause headaches or, if things go really wrong, worse health problems. Anyone painting their house or using a solvent-heavy cleaner should open windows and step outside for a break.
There’s also the big question—what happens after a product with butyl propionate gets tossed out or washed down the drain? Studies have looked at how this chemical breaks down outside and in wastewater plants. Good news: it does tend to degrade before it causes long-term harm, but in big enough doses, it has caused trouble for aquatic life or the air right above where it gets released. This is one of those reminders to use as much as you need, and not more. In my early days helping repaint my family’s house, I learned pretty fast to cover up, keep the windows open, and save every last drop—partly to avoid wasting money, and partly because of that sharp smell reminding us these products aren’t harmless.
Folks watching the chemical industry have started seeing more alternatives pop up; paints and cleaners with fewer solvents or made from plant-based sources grab attention. Still, many businesses stick with butyl propionate because it’s reliable, affordable, and makes products work better. Even so, plenty of us—myself included—now try to read labels, pick safer versions if possible, and handle any solvent with care. It’s not about banning every chemical, just about being smart so today’s fixes don’t turn into tomorrow’s new headaches.
Most people don’t go through life thinking much about Butyl Propionate, but that doesn’t mean it’s not kicking around somewhere nearby. The chemical formula for Butyl Propionate is C7H14O2. That short string of letters and numbers packs a story you’ll find in the perfumes people spray on in the morning, or even some paints used for weekend home projects.
Butyl Propionate belongs to the family of esters. You might remember esters from high school chemistry—those compounds that usually smelled a bit fruity. Scientists make this one from butanol and propionic acid, and the result brings together durability and a pleasant aroma. That’s a combo folks working in labs and industries chase all the time.
For those not living and breathing molecular models, the formula breaks down like this: seven carbon atoms, fourteen hydrogen atoms, and two oxygens glued together in a way that creates an ester. This specific shape sits at the root of its ability to dissolve different ingredients, help paints dry just right, and give off that signature clean, sweet scent.
Thinking about how this matches up with uses in daily life opens a window into why certain products work better than others. In college, some of us kept our dorm windows open because the art students two doors down used solvents with a sharp chemical bite. Butyl Propionate, though, doesn’t leave the same harsh impression—and that sort of detail can matter a lot for people sensitive to strong odors.
No matter how useful something seems, there’s always a question about safety. C7H14O2 can catch fire, so storing it right is key. Working around it often means smart ventilation and good gloves. It evaporates fairly quickly, leaving less residue than other chemicals, but even that benefit means people need to watch for vapors in closed spaces.
Regulators don’t let just anything make it onto shelves or into workplace inventory. Butyl Propionate carries safety data warnings, and responsible manufacturers publish those so workers and customers know what they’re dealing with. In my own experience at a friend’s workshop, just reading the labels before opening paint made a difference. Understanding what “flammable” really means—especially if you’ve seen a small chemical fire up close—reminds you to take recommendations seriously, not as suggestions.
Chemicals like Butyl Propionate highlight bigger questions about how we balance performance with health and environmental impact. Right now, producers and scientists push for alternative solvents and new formulations every year, aiming for the same reliability without leaning on fossil-based ingredients. Finding replacements that get the right performance out of coatings, adhesives, or fragrances without adding to pollution or exposure risks won’t come easy.
What helps is honesty in labeling and innovation that doesn't just reinvent something for the sake of a patent, but really makes it safer. The people working with these chemicals every day—at factories, in auto body shops, in small art studios—deserve to breathe easier and stay healthy, without warning lights always blinking in the back of their minds.
Butyl propionate pops up in industrial spaces, paint booths, and even some cleaning products. It has a fruity, pleasant smell, so you might assume it’s harmless. Plenty of folks feel if a chemical doesn’t stink or burn the eyes, it can’t do much harm. But that’s not always the full story, and butyl propionate deserves a closer look for both workers and regular folks.
From daily experience around paints and solvents, I know it’s the invisible hazards that cause most trouble. Butyl propionate vapors may seem subtle, but breathing them in for hours creates problems down the line: headaches, dizziness, and throat irritation show up fast. On job sites with poor ventilation, those symptoms hit even faster. Some factories keep windows shut tight, trusting that regulations mean chemicals must be safe. Yet after stripping paint or spraying coatings, I’ve watched coworkers struggle to focus or feel outright sick. When that keeps happening, it’s time to take a step back.
Reports in safety journals point out high exposures can even depress the nervous system. My own rule, based on plenty of afternoons spent fighting nausea in stuffy workshops, always steers me toward better airflow and breaks outside. Using gloves and eye protection helps, too, since even skin contact invites irritation. You might not notice redness right away, but after hours of handling rags soaked in solvents, the sting and dryness can’t be ignored.
Short-term discomfort tells only part of the story. The concern for those working with butyl propionate day after day rests on chronic effects. Regulatory groups like OSHA and NIOSH track dangerous exposure levels, but in practice, personal protective gear often ends up neglected unless the boss insists. Over years, constant low-level exposure can pile up, leading to more serious nervous system symptoms, deeper respiratory troubles, or lasting skin sensitivity. It’s easy to forget about those risks until they become obvious, but by then, damage lingers.
Many workplaces still treat solvents as “everyday” hazards, like noise or dust, instead of something that calls for careful respect. That attitude trickles down into how seriously rules get followed. Even with safety data sheets available, the warnings fade into the background. I’ve seen safety meetings skim right past chemical hazards unless someone presses the issue.
Better solutions start with honest talk about what butyl propionate can do. On job sites, that means not just telling folks to use protective gear, but showing what bad exposure actually looks like. Strong ventilation matters more than fancy monitoring equipment. Open windows, working fans, and time spent outdoors between tasks make the biggest difference, at least in my own work life.
On the manufacturing side, engineers could rethink formulas to swap in safer solvents where possible. Industries shifted away from lead in paint for a reason—no need to wait for disaster before making another change. At home, picking low-VOC or “natural” cleaning products keeps families away from unnecessary risks.
Butyl propionate leaves a clear lesson: just because a chemical smells sweet or feels mild, it can still leave a mark over time. Good habits protect health, whether you’re spraying paint in a maintenance shop or wiping down your kitchen. Listening to how bodies react, pushing for better worksite habits, and pressing companies toward safer ingredients create protections that last far beyond this week’s to-do list.
Butyl propionate has a reputation for being volatile. Anyone who’s handled industrial solvents for more than a few weeks knows the difference between hopping from spill to spill and actually storing something properly. If a chemical has a strong smell and can catch fire easily, sloppy storage is a bad gamble.
Lay out the basics right from the start—keep butyl propionate containers in a cool spot, far away from open flames and heat sources. No one ever regretted having a separate, ventilated room with a concrete floor for solvents. Metal drums and tight-sealed containers matter here, not some old plastic jug. Never assume you’ve closed it tight enough—double-check every time. One leaky drum can be all it takes for that sharp odor to fill the air and headaches to set in.
Every solvent brings a fire risk, but butyl propionate comes with a low flash point. Sparks from electric tools, static from plastic wrap, even warm bulbs are enough to kickstart a bad day. A decent fan and a good extractor give fumes somewhere else to go rather than drifting through the air and into someone’s lungs.
Workplaces that treat chemicals casually usually end up with someone sick or injured. Set up proper electrical grounding for drums and pumps—those details keep static from building up. You won’t see static coming, so don’t let it become the hidden danger it usually is.
Use the original label, and don’t move butyl propionate into an unmarked bottle just for convenience. Emergency crews need to know what spilled if something goes wrong. One shop used to pride itself on “common sense” handling, only for an employee to pour butyl propionate into a water bottle, thinking it was fine for storage. That turned into a panic when someone reached for what looked like a drink.
Clear labeling is more than just a rule—it’s basic decency to your coworkers. Keep safety data sheets in the same room, not tucked away in a binder in another corner of the building. In an emergency, those seconds matter.
Personal protection isn’t glamorous, but gloves and goggles save you a trip to the clinic. After handling butyl propionate, the last thing you want is a skin rash or red, watering eyes. I’ve seen one too many folks skip the gloves because they “just needed to grab something quickly.” Days later, they show up with peeling skin, blaming bad luck. Take five extra seconds, grab your gear, and go home with healthy hands and eyes.
Leaking drums, spills, accidental mix-ups—no one expects them, but they always happen. Absorbent spill kits should be on every floor. Crews should know exactly what to do if something tips over. Fresh air, fast response, and honesty about what happened keep accidents from turning into disasters.
Clear up any spill as soon as it happens. If it soaks into cardboard or wood, all that’s left is to replace the contaminated materials. Trying to “air things out” doesn’t work with butyl propionate. It’s helpful to walk through what-if scenarios with your team—everyone remembers better in practice than under pressure.
Butyl propionate has useful properties, but it doesn’t take kindly to shortcuts. Simple steps like using proper storage, solid labeling, keeping up with safety training, and having spill-control tools close by actually make a difference. I’ve seen workplaces transform just by getting the basics right—less drama, fewer sick days, and safer, happier staff.
You can spot butyl propionate in quite a few places, sometimes hidden in plain sight. Though most folks never hear about it, anyone working with paints, coatings, or ink formulations probably gets a whiff on a regular basis. Clear and colorless, this organic liquid slides out of the bottle with a kind of understated confidence, never thick or sticky. You give it a swirl and see how easily it moves — pretty fluid, not syrupy at all.
People pay a lot more attention to butyl propionate’s chemical antics, but its physical properties set the stage for safe handling and efficient use. Just over the density of water, weighing in around 0.87 grams per cubic centimeter, it feels noticeably lighter in the hand than glycerin or most oils. This low density makes transferring, mixing, or diluting it a bit easier — less weight to drag around, less mess after a spill, and faster cleanups.
Most folks care if a liquid is going to heat up the shop or cool it down. Butyl propionate’s boiling point sits at roughly 146°C (about 295°F). So, it doesn’t steam away into the air at room temperature. That sort of stability opens doors for precise drying times in paint shops and consistent output in printing plants, and keeps people from worrying so much about losing product to evaporation during storage.
A big part of the experience with butyl propionate comes down to its smell. Fruity, kind of sweet — it lingers in the air much longer than you'd think. I once spent an afternoon repainting a couple office doors with a product that used butyl propionate as a solvent, and ended up smelling that faintly pear-like scent every time I walked by for weeks.
For good or bad, butyl propionate doesn’t love mixing with water. Its solubility floats around 1.5 grams per liter, so it mostly shrugs off a water rinse. On the flip side, it happily combines with most common industrial solvents, like alcohols or ketones. This lets manufacturers tweak their recipes to fit the needs of a job, from adjusting drying speeds to finding that sweet spot on gloss and leveling in coatings.
Potency matters in both the science lab and the paint shed. Butyl propionate vapor brings mild flammability risks to the table — a flashpoint of roughly 39°C (around 102°F) draws a clear line for safe storage and usage. In the humidity of a southern summer, that’s close to uncomfortable indoor temperatures, so proper ventilation and spark control feel less like optional steps and more like common sense.
No one wants to risk headaches or exposure. NIOSH classifies it as an irritant in concentrated amounts, so splashing it around without gloves or a mask brings stinging eyes, headaches, or worse. The liquid runs off skin quickly but clings to porous surfaces. Good gloves and goggles are part of the tool kit — not some afterthought at the end of a safety sheet.
Instead of only worrying about production numbers, shops in my area started swapping in vented mixing rooms and spill-proof containers after a few close calls with solvents. More attention landed on regular training, not just for chemical engineers but for anyone pushing a mop or wiping up brushes. By keeping butyl propionate locked up away from direct heat and steadying the room’s temperature, the number of spills and accidental vapor releases dropped noticeably.
Looking at waste disposal, local guidelines highlight the need to keep waste streams labeled and separated, since this solvent finds its way into landfills only after lots of oversight. Switching to less volatile alternatives sometimes means giving up speed or finish quality, but safety and peace of mind aren’t measured on a production line. Every property of butyl propionate — from how quickly it evaporates to how it smells — creates ripple effects for safety plans, maintenance costs, and workplace comfort.
Plenty of chemicals fade into the background of daily life, butyl propionate included. Maybe the name sounds intimidating, like something buried at the bottom of a safety data sheet, but its fingerprints show up in places you wouldn’t expect. Over the years, I noticed that the fresh smell of nail polish, the quick-drying touch of paint, and the easy swipe of a cleaner all share something. Each owes its slick performance to ingredients like butyl propionate.
Open a can of high-gloss paint or reach for a quick-drying varnish, and butyl propionate is doing some quiet heavy lifting. It works as a solvent, helping those paints flow onto surfaces smoothly. Without it, brushes would drag, rollers would stick, and the finish would never look right. If you’ve ever tried scrubbing sticky paint off your hands, you know why a proper solvent matters. Painters and DIY types save hours because compounds like this speed drying times and improve spread-ability. Less downtime means quicker renovations and lower labor bills.
People love the scent of fresh nail polish or a just-wiped countertop. Butyl propionate has a faint, fruity odor that does double duty. It covers up harsh base smells in cosmetics and polishes, helping nail salons not reek like acetone factories. At home, surface cleaners pick up a whiff of fruitiness from this chemical. It’s these touches that keep a home or salon from feeling like a lab.
Manufacturing never stops, and butyl propionate shows up on big production lines too. Adhesives and inks flow better with its help. Printers and glue manufacturers prize it for its ability to make sticky substances manageable and easy to spread. Working in packaging years ago, I saw how a label needed the right touch—too sticky, and you deal with jams; too wet, and it peels off. Getting things balanced kept us moving at pace.
No chemical is perfect. Butyl propionate, like all solvents, demands respect. In manufacturing, people handle it with gloves and ventilation systems. Its low smell makes overexposure sneaky; you don’t always realize fumes are present, which underscores how important safety rules become in shops and factories. Government guidelines call for careful storage and airflow, not because anyone enjoys extra expense, but because regular contact can lead to headaches or eye irritation. I recall a time working with paints inside a poorly vented space—it took just an hour to get a solid headache, a lesson hard to forget.
Demand for safer and greener chemicals grows every year. Some companies swap out solvents like butyl propionate for alternatives made from renewable resources. Research into plant-based substitutes or water-based formulas picks up as regulations tighten and customer preferences change. It’s not always simple—performance counts for plenty in paints, adhesives, and cleaners. The real challenge becomes finding something as effective as butyl propionate that also keeps both workers and the planet at ease.
Butyl propionate won’t disappear overnight. It has built a quiet reputation for keeping things neat, glossy, and pleasant-smelling. But like so much else, it asks us to balance convenience, safety, and progress. That’s what keeps the conversation alive in every industry where performance counts, but well-being matters most of all.
Walk into any paint shop or chemical warehouse, and at some point, you’ll run into butyl propionate. It's a clear liquid used in coatings, inks, and cleaning products because it dissolves substances that water leaves behind. Some folks unfamiliar with it might treat it like just another bottle on the shelf. That sort of thinking can land you in trouble.
Butyl propionate carries risks many don’t see coming. The smell alone can set off headaches or dizziness if the space gets stuffy. Spilled on skin, it can irritate, and the liquid can sneak into cuts or scrapes. Left uncapped, it evaporates and adds fumes to the air you breathe. In the right conditions, the vapors can even catch fire. These dangers might seem distant until someone shakes the can for too long or leaves rags soaking in a corner.
Putting on the right clothes does half the work. I keep a pair of safety goggles handy and reach for gloves before even popping the lid. Less exposed skin means fewer problems, especially if the job goes sideways. Good gloves—nitrile beats latex every time—stop the stuff from soaking into your hands.
Once, after I forgot my gloves, a single splash left my hand red for days. The lesson stuck. Superficial burns might heal, but why take the gamble?
Ventilation makes a bigger difference than folks realize. Opening a single window in a tight room won’t cut it. Setting up a fan to move air outside pays off, especially in garages or basements where fumes settle. The less I smell, the better I feel after the work’s done. In some cases, a simple dust mask won’t protect lungs from chemical fumes, so a proper respirator rated for organic vapors offers real peace of mind when handling the stuff indoors.
Mistakes with storage often end in panic. Butyl propionate needs a solid, tightly sealed container. Metal or heavy-duty plastic works—just check for labels that list compatibility with solvents. I never store this chemical near heat sources. A hot light bulb or set of work tools can do real damage if leaks happen.
Spills call for paper towels or absorbent material made for chemicals—kitty litter in a pinch. Afterwards, I bag everything tightly and keep it out of regular trash bins. Many local waste centers have clear rules about getting rid of solvent-soaked rags. Ignoring those rules courts disaster, from garbage fires to environmental problems. Just last year, a local workshop caught fire because workers tossed solvent rags into an old trashcan. The aftermath left the building shut for weeks.
It’s easy to think these safety steps slow things down. But the facts back up their value. The National Institute for Occupational Safety and Health reports that exposure to solvents like butyl propionate can cause long-term issues, from nerve damage to organ stress, if people get too casual. Even for those working at home, it only takes one mistake to cause lasting harm. Posting warning signs in shared spaces and double-checking ventilation routines protect more than just the person pouring from the can.
Safer habits grow out of honest conversations with experienced coworkers and a willingness to read real-world stories—not just the small print on a label. Respect for the risks promises easier workdays and fewer trips to the emergency room. That’s a trade-off anyone handling butyl propionate should make.
Stepping into a chemistry lab, colorful bottles line shelves with labels written in a language that takes a bit of patience to crack. Butyl propionate is one of those names that brings a technical ring, yet this simple ester finds a place well outside the lab, often popping up in everyday products like paints and coatings because of its pleasant smell and great solvent ability.
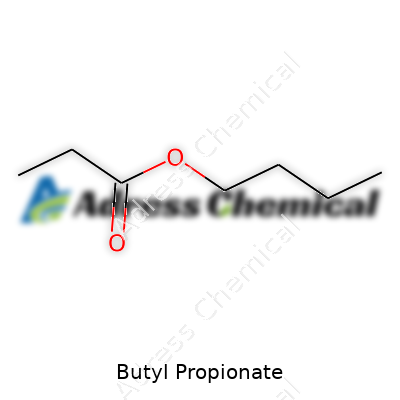
Butyl propionate stands for a chemical compound that brings together two groups: butyl, which comes from butanol, and propionate, made from propionic acid. Bringing these two together forms an ester. It comes with the chemical formula C7H14O2. Each bit of the formula reflects something—seven carbon atoms, fourteen hydrogens, and two oxygens.
If you stack the molecule flat in your mind, the “butyl” piece is a chain with four carbons, hanging off a backbone made by the three-carbon “propionate.” The chemistry doesn’t look fancy, but it’s useful. Chemists write it out as CH3CH2COO(CH2)3CH3, expressing its joined-up parts.
Working with chemicals in the real world means weighing and mixing, not just talking formulas. Molecular weight matters because it tells you how much a mole of molecules will weigh—a basic measure straight out of any prep room in a factory or research site. For butyl propionate, that number comes out at about 130.18 grams per mole. That’s not just trivia. This number decides how much ends up in mixing tanks, how fumes spread through the air in a paint shop, and how easily the stuff can be packaged and shipped without running into safety headaches.
You can’t just glance at a bottle and know it’s butyl propionate. Quality control and regulation in the chemical industry depend on exact numbers. Mixing up formulas or weights can lead to wasted batches or, in tougher cases, health risks at the workplace. Nailing down the chemical formula and the right molecular weight keeps engineers, artists, and technologists on the same page.
It makes sense to lean on better chemical education and hands-on practice with measuring tools. Young chemists and technicians should get plenty of time learning how to double-check these basic facts—formulas, structures, weights—before setting foot in a production plant. The gap between knowing what a molecule does on paper and how it acts in a drum gets smaller with good training and real oversight.
On top of that, tighter labeling rules and accessible information in workplaces improve safety and help prevent mistakes. Easy-to-read sheets explaining formulas and weights can live right next to the barrels, not just in office binders. Small efforts like these keep a simple ester like butyl propionate from causing bigger headaches, letting everyone keep focus on results.
Anybody searching for a quick guide on storing butyl propionate might feel tempted to treat it like any household solvent, but its story runs deeper. This isn’t just another clear liquid that can sit forgotten on a shelf. Butyl propionate has a knack for evaporating fast and catching fire even faster. Left uncapped or near warmth and a single spark, it’ll show very little hesitation. I remember once cleaning out a shared laboratory space, finding a bottle of butyl propionate sitting right next to a toaster oven. Whoever left that bottle behind had no sense of timing—or safety. I learned early that nothing good comes from mishandling volatile chemicals.
Butyl propionate reacts poorly with heat. Leaving it in a sunlit window or in a stuffy room invites trouble. Any place where temperatures surge creates a risk—not always dramatic, sometimes just insidious leaks, strong smells, or slow spoilage. I’ve seen storage rooms where a lack of basic ventilation led to headaches and complaints. Good airflow and moderate temperatures save people and product. Even labs with modern systems often add extra fans or just keep stock in a refrigerator set to chemical storage standards. Fancy setups help, but respecting the basics—shade and coolness—beats high-tech gear every time.
One rule stuck with me from the first day I worked around chemicals: always check the cap. Some folks leave a solvent half-capped, thinking they’ll return soon. Butyl propionate punishes carelessness by sending vapors into the air or leaking right through a loose lid. Strong containers with good seals keep fumes inside, prevent spills, and limit fire risk. Glass bottles work best where possible, followed by heavy-duty plastic made to withstand aggressive chemicals. Lids need to fit tight. Try storing it in thin plastic, cheap jars, or unlabeled bottles, and you’ll find that easy choices make the biggest messes later.
Most old labs make a clear point: label everything. A hand-written scrawl that says “danger: butyl propionate, flammable” isn’t paranoia, it’s practical. It stops anyone from thinking it’s water or something harmless. I’ve seen folks mix up bottles due to lazy handwriting or smudged labels, only to realize the error during a crisis. Keep signs clear, make them bold, update them after every refill.
And then there’s the classic fire hazard. Sparks don’t care if you’re distracted, so keep butyl propionate far from open flames or running electrical equipment. Use spark-proof switches, ground all gear, and make it a routine to double-check—especially late in the day when people get careless.
Storing butyl propionate brings up the human factor. Routine moments—topping off bottles, moving containers, taking inventory—can trip up anybody. Policies matter but habits matter more. Training new staff, double-checking with lists, and holding short safety meetings helped catch most slip-ups. Spills will still happen, so keeping absorbent pads and a clear route to fresh air gives everyone more breathing room. Plenty of folks see this prep as overkill until the day it pays off.
Simple fixes make the difference. Pick a cool, ventilated spot. Grab sturdy containers and check them often. Mark every jug clearly. Pass on a little chemical respect, especially to anyone new on the job. It’s easy to cut corners or trust luck, but mistakes around butyl propionate don’t end quietly. Keep things neat, don’t rush, and treat each bottle like it deserves your full attention.
Butyl propionate hardly rings familiar for most people. Yet, it finds its way into paints, coatings, inks, and some cleaning products. It's got a fruity smell, almost pleasant, masking the potential risks it brings along. Since paint fumes always hit my nose whenever I walk past a construction site, I started reading the label details. Butyl propionate, as simple as it sounds, deserves a closer look, especially as more people try their hand at DIY home projects.
Strong-smelling solvents practically wave warning flags. Inhaling butyl propionate may lead to dizziness or headaches quite quickly. Spend a few hours in a small room using a paint product containing this chemical, and you might end up feeling queasy or a bit light-headed. Eye or skin contact also tends to cause irritation, sometimes worse if there's not enough airflow. Some people have tougher reactions, ending up with serious respiratory issues from repeated exposure.
The Occupational Safety and Health Administration recommends proper ventilation and gloves, not just for show. Even a quick task can build up enough fumes to hurt your lungs. The trick is not just opening a window when handling products with butyl propionate but thinking smart about protection—ventilated masks, fans, and, for professionals, real extraction systems make all the difference.
Short-term symptoms like dizziness and irritation can serve as warning signals, but concerns around chronic exposure strike a deeper chord. There isn't as much documented research about lifelong risks from butyl propionate as there is for some big-name solvents, but that doesn’t mean it's safe. Workers in industries using solvents day after day sometimes end up with long-term nervous system problems, including memory issues, trouble focusing, and mood swings. Even without headline-grabbing studies, erring on the side of caution makes sense. If I were sanding, painting, or cleaning up in a garage, I wouldn't dismiss those lingering odors or persistent headaches.
Once butyl propionate evaporates, it heads outdoors, where it breaks down in sunlight in a matter of hours to days. At first, this might seem unimportant—out of sight, out of mind. Still, the story doesn’t stop there. Large-scale releases or careless spills can threaten fish and aquatic plants. This stuff doesn't last long in water, but even a short visit causes harm to delicate gills or roots. The United States Environmental Protection Agency lists it as a volatile organic compound, which plays into smog formation, especially during hot summer afternoons in crowded cities.
Fighting chemical hazards requires more than just worrying—choices matter. Swapping old solvent-heavy products for water-based paints and cleaners can make a big dent in everyday exposure. Proper disposal stops the spread, too. Pouring leftover solvents down the drain sounds all too common but puts local waterways at risk. Dropping off leftovers at community hazardous waste centers makes sure they don’t end up in rivers or soil.
Whenever I pick up a can of paint or a bottle of cleaning fluid, I take a moment to scan the ingredients. Taking simple steps—good airflow, gloves, and careful handling—turns risky jobs into safer routines. If more folks start asking about what’s in their products and demanding clearer labels, safer alternatives will end up on the shelf. Everyone benefits from that, both at home and around the neighborhood.
| Names | |
| Preferred IUPAC name | Butyl propanoate |
| Other names |
Propionic acid butyl ester Butyl propanoate Butyl n-propionate n-Propionic acid butyl ester Butyl propionate |
| Pronunciation | /ˈbjuːtɪl prəˈpɒneɪt/ |
| Identifiers | |
| CAS Number | 590-01-2 |
| Beilstein Reference | Beilstein 1721533 |
| ChEBI | CHEBI:88457 |
| ChEMBL | CHEMBL48663 |
| ChemSpider | 7711 |
| DrugBank | DB16584 |
| ECHA InfoCard | '100.034.795' |
| EC Number | 203-689-4 |
| Gmelin Reference | Gmelin Reference: **78680** |
| KEGG | C19268 |
| MeSH | D017850 |
| PubChem CID | 8019 |
| RTECS number | ES5425000 |
| UNII | 2D587JAI5E |
| UN number | UN2348 |
| Properties | |
| Chemical formula | C7H14O2 |
| Molar mass | 130.19 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | fruity |
| Density | 0.874 g/cm³ |
| Solubility in water | 0.75 g/100 mL (20 °C) |
| log P | 1.94 |
| Vapor pressure | 0.5 mmHg (20°C) |
| Basicity (pKb) | 15.43 |
| Magnetic susceptibility (χ) | -7.58×10⁻⁶ |
| Refractive index (nD) | nD 1.406 |
| Viscosity | 0.62 mPa·s (25 °C) |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 249.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -471.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3585.7 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| Flash point | 33°C |
| Autoignition temperature | 370°C |
| Explosive limits | 1.1% - 7.0% |
| Lethal dose or concentration | LD50 (oral, rat): 10930 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 9740 mg/kg |
| NIOSH | BNN |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 300 mg/m³ |
| IDLH (Immediate danger) | IDHL: 1700 ppm |
| Related compounds | |
| Related compounds |
Ethyl Propionate Methyl Propionate Propyl Propionate Butyl Acetate Butyl Butyrate |