Butyl glycolate didn’t spring up overnight. In the early to mid-20th century, increased demand for specialized chemicals in coatings, plastics, and cleaning shifted chemists’ attention toward glycol derivatives. Among them, butyl glycolate caught the eye for its solvent power and decent balance of volatility and stability. Major chemical producers experimented with esterifying glycolic acid using butanol through acid catalysis or Fischer esterification, and that process hasn’t changed much since. By the end of the century, global industries were pulling butyl glycolate into more and more applications, driven by the need for solvents and intermediates that balanced performance with manageable safety profiles.
Butyl glycolate holds a position as a handy glycolic acid ester, typically showing up as a colorless to pale yellow liquid, featuring that telltale ester smell. Chemists and producers rely on its solvent power for everything from resins and lacquers to specialty cleaning ingredients and coatings. Compared to heavier or more volatile esters, butyl glycolate handles well, mixes smoothly with a range of organic solvents, and finds some favor wherever gentle solvency and controlled evaporation make a difference.
Most folks working with butyl glycolate learn quickly it boils right around 189–191°C and sports a flash point near 80°C, putting it a few safety steps ahead of other ethers or esters that race to ignite. Its specific gravity floats around 1.01 at 20°C, showing it settles into water just fine, yet its water solubility stays low. It dissolves in ethanol, ether, and similar organics. On the chemical side, it resists hydrolysis fairly well at neutral pH but starts breaking down in stronger acid or base, giving back glycolic acid and n-butanol. This dual nature makes it valuable both as a working solvent and as a synthetic intermediate.
Producers typically list butyl glycolate with a purity not less than 98%. Color guidelines often insist on APHA values under 30. Water content needs to stay below 0.2%, and non-volatile residues should fall under 0.05%, as instrument calibration can detect even tiny impurities. On drums and labels, expect clear hazard warnings—flammability, mild eye or skin irritation risks, and handling instructions in line with GHS and local worker safety laws. Labs and production lines who ship this ester follow UN1993 guidelines, not just for legal reasons but to keep people and transit safe.
Getting butyl glycolate into a drum starts from glycolic acid and n-butanol, with a dab of acid catalyst. Most syntheses lean into Fischer esterification: reflux glycolic acid with n-butanol in the presence of acid, usually sulfuric, all while managing water removal so the reaction climbs toward completion. Continuous removal of water helps due to the equilibrium nature of esterification. Afterward, the batch gets neutralized, then distilled under reduced pressure to pull off pure butyl glycolate. Some tech groups in industry have nudged yields up and waste down by tinkering with catalysts, stripping columns, or even using enzyme methods for greener chemistry, but the backbone stays rooted in classic acid-catalyzed esterification.
In lab beakers, butyl glycolate stands up to plenty but starts to hydrolyze when hit with real acid or base. Add water and an alkaline catalyst, and it revs back to glycolic acid and butanol, a trick useful for reclaiming value downstream. As an ester, it partners well in transesterification, letting chemists swap out the butyl group under the right conditions. Some research teams tinker with its backbone, aiming to tailor volatility or solvent power by introducing new alkyl groups. It doesn’t join radical polymerizations easily, limiting use in plastics, but acts as a softer modifier in acrylic resins and select copolymers where compatibility and surface finish matter more than high-speed chemistry.
Depending on where you buy it, butyl glycolate turns up as n-butyl glycolate, butyl-2-hydroxyacetate, or butyl hydroxyacetate, and a few catalog listings list 'glycolic acid butyl ester.' Chemical supply houses and big distributors push their own product numbers or brand naming, but for most users, the CAS number 7397-62-8 cuts right through the clutter. This sort of aliasing sometimes confuses purchasing or inventory teams, so regulatory and EH&S departments ask for precise naming on documents and labels.
Butyl glycolate handles pretty well if you respect its solvency and mild volatility. Safety data points to eye and skin irritation from direct contact; vapor at high levels can irritate the upper respiratory tract. While its flammability runs less aggressive than ethers or ketones, open flames, sparks, or hot surfaces still pose real risks. Facilities working with butyl glycolate demand good HVAC, splash-proof goggles, gloves, and ready spill kits. OSHA standards push exposure limits low, and European REACH data signals a moderate safety tier compared to heavier solvents. Proper training heads off most incidents, but regular safety refreshers and strict control over storage conditions keep accidents rare.
It’s in the coatings space where butyl glycolate finds regular action, loosening pigments, letting resin blends level out, and helping water-based or hybrid coatings dry at a steady clip. Paint makers like it for streak-free finishes, and cleaner formulators blend it with other glycolates or hydrotropes for tough grease or organic soil removal. Some formulators tap it for cosmetics or personal care, but only after careful review of safety and purity; even then, use stays limited. In textile work, it helps in specialty finishes and cleaning baths, where solvents need to pack a punch without wrecking delicate fibers. Even in electronics, certain cleaning or degreasing baths build in butyl glycolate as a compromise between power and gentleness.
Across university and industry labs, researchers keep tinkering with butyl glycolate both to understand its limits and to extend its reach. Analytical chemists probe its performance in new solvent blends, hoping for safer substitutes to traditional glycol ethers. Green chemistry groups test bio-based glycols and butanols, aiming for lower-carbon esterification, while engineers tweak manufacturing routes to drop energy and waste. Computational chemists dig into its dissolution mechanics, eyeing performance boosts for specialty coatings or cleaners. A handful of projects dive into modifying its ester group, with the hope that new alkyl or glycol backbones will carve out new uses in additives or surface treatments. Most advances stay close to cleaner, safer, and more effective performance for practical chemical applications.
Most published animal studies position butyl glycolate as a mild irritant. At high doses, it can trigger transient central nervous effects in rodents, but test protocols flag wider margins of safety for common industrial uses. Long-term tests don’t show a major risk of mutagenicity or reproductive issues, but regulatory reviews press for ongoing monitoring, especially with stricter EU REACH and US EPA oversight. In talking shop with regulatory experts and toxicologists, the consensus lands somewhere between “cautious respect” and “routine industrial vigilance.” Product stewardship groups keep current on workforce exposure data, chasing better safety practices and transparent risk assessments.
Butyl glycolate’s future looks steady, though not explosive. Downstream demand rides on coatings, cleaning, and chemical processing, so as industrial users hunt for greener solvents and flexible intermediates, butyl glycolate stays in the running. Some pressure shows up from bio-based and safer alternatives, but supply chains and costs still give it a solid lead. Researchers keep poking at all angles—a push for better purification, greener chemistry, and expanded applications in resins and cleaners. If regulatory bars rise on glycol-based esters, chemists may need to nudge butyl glycolate into safer, lower-impact roles, or even reinvent its backbone entirely. The watchwords for its longevity are smart stewardship, continued R&D, and honest engagement with both safety data and evolving industry needs.
Walk through any hardware store, check out the cleaning aisle, or read the fine print on labels at a paint supply shop—chances are, you'll find butyl glycolate listed. This chemical doesn't grab headlines, but it quietly makes big jobs easier, safer, and more efficient in homes and factories. I learned this when I worked in a paint shop during college and got an up-close look at how small components in a formula can change everything about how a product works.
Butyl glycolate shows up a lot in cleaning products. It acts as a solvent that helps dissolve dirt and greasy grime that water alone can't tackle. Many store-bought floor cleaners, window sprays, and degreasers rely on chemicals like this to get results that elbow grease alone can't achieve. When mopping up after a holiday party or scrubbing kitchen gunk, I've seen how floor cleaners cut through messes in a way plain soap and water never could. That power often comes from solvents like butyl glycolate working behind the scenes.
Manufacturers add butyl glycolate to paint and coatings so that thick liquids glide smoothly onto walls or furniture. Drying too quickly leads to streaking or uneven color, but this chemical helps slow the process just enough. As someone who spent days repainting old apartment walls, I appreciated paint that leveled out and dried evenly. It's a small thing that saves time and money for both do-it-yourselfers and pros. The chemical structure of butyl glycolate allows it to blend well with pigments and resin, so paints stay consistent and don't harden too fast. Some formulas even depend on this chemical for better adhesion to difficult surfaces.
Big factories use butyl glycolate, too. Textile processors rely on it to clean fibers before dyeing fabric, since stubborn stains or leftover oils can block dyes and produce patchy results. In metalworking, companies use it as a solvent to remove unwanted residues from metal parts before plating or coating happens. During one visit to a relative who worked at an auto factory, I watched how cleaning agents worked on greasy car parts coming off the assembly line. That deep clean—essential for quality control—relies on chemicals like butyl glycolate in the mix.
Working with chemicals means paying attention to risks. Companies and workers must handle butyl glycolate with care. Skin contact or inhaling fumes can lead to irritation, so wearing gloves and working in well-ventilated spaces makes a big difference. I've learned through experience the importance of reading labels and following workplace guidelines. Product manufacturers post safety data sheets and use warning symbols to help keep users informed about handling and first aid tips. Responsible storage—away from heat or open flames—matters in both homes and industrial settings.
The biggest reason butyl glycolate stays popular comes down to performance. It boosts cleaning strength and helps paints look better. But there’s growing demand for safer, greener options. Some companies now test plant-based solvents or biodegradable blends as substitutes. Research continues to check for long-term effects and to cut down on environmental footprint. As someone who prefers to limit harsh chemicals at home, I keep an eye on product developments. That way, we all get clean homes and great paint jobs—without risking our health or the planet.
A trip down any beauty aisle leaves you squinting at bottles, tracing the ingredient lists with your finger. Butyl Glycolate—a name that rolls about as easily as the texture it brings to lotions and creams—shows up more than people might realize. Coming from hands-on experience with formula testing and product research, I’ve always made it a point to trace not just what an ingredient does, but what it means for anyone putting that cream or serum on their skin. Safety concerns shouldn’t get buried behind a label.
This compound works as a solvent. It thins things out, helps other ingredients blend, and can enhance texture in a finished product. Cosmetic chemists see it as a go-to for keeping products smooth and easy to spread. But a product feeling nice doesn’t answer the real worry many folks have—what does it do to your skin, and what risks come along for the ride?
Cosmetic ingredient safety checks don’t run on guesswork. In the United States, the Food and Drug Administration and independent panels like the Cosmetic Ingredient Review keep a close eye on common compounds. For Butyl Glycolate, data shows relatively low toxicity at the concentrations used in skincare and personal care items. Animal studies looked at repeated exposure and saw no buildup in tissue or signs of severe irritation. Human patch tests echo these results—folks applying test formulas daily for weeks rarely experienced more than a slight tingle if anything at all.
People with sensitive skin still face a question mark. As with fragrances and other solvents, a tiny percentage get redness, itchiness, or flaking. Over a decade in product development, I’ve learned to listen to real customer feedback. Allergy reports turn up, but rarely, and usually only for those who tend to react poorly to a whole set of ingredients, not just Butyl Glycolate alone.
Trust grows from a mix of science, transparency, and lived experience. Ingredient listings tend to read like a foreign language to most shoppers, but not for lack of care—it’s just that keeping formulas stable, effective, and shelf-safe means reaching for reliable chemicals. No one in the business wants a recall from irritation, lawsuits, or lost trust. I’ve met many brand owners who triple-check the latest safety data and reformulate at the first sign of concern.
Sustainability and health concerns are changing the conversation. Many shoppers want shorter lists, less jargon, and more proof that the stuff going on their skin won’t cause long-haul damage. Trustworthy brands publish numbers, cite studies, and avoid hiding behind “proprietary blends.” My advice for shoppers: look up new ingredients on the European Chemicals Agency database, read the Cosmetic Ingredient Review reports, and email a company if something doesn’t sound right.
No chemical brings zero risk, especially for those with sensitive or allergy-prone skin. Patch testing new products isn’t old-fashioned advice—it’s essential. Reading recent safety reviews and sticking with brands that disclose their ingredient sourcing makes a difference. Regulations matter, but so does taking control of your routine and speaking up about reactions.
Bottom line, the science shows Butyl Glycolate is among the safer choices found in modern skincare. Paying attention, asking questions, and learning from both science and real people’s stories keeps skin health at the center of beauty, where it belongs.
Most folks don’t realize that butyl glycolate sits in the background of a lot of every day products. If you’ve scrubbed a stubborn stain from your kitchen tile, or picked up a bottle of household cleaner, there’s a good chance this clear liquid played a hand. Companies pick it for its ability to break down greasy messes and keep solutions from separating. It shows up often in paints, adhesives, and even some polishes.
Butyl glycolate stands out because it carries a scent similar to fruit and withstands quite a bit of heat without breaking down. It holds a spot in a family of chemicals called glycolates, which combine alcohol and acid in one small molecule. A lot of solvents tend to push away water, but butyl glycolate gets along with both water and oil. That makes it valuable when working with products that ask for a balance, such as some cleaning fluids or coatings that must resist streaking or drying out.
With strong cleaning power comes some caution. Butyl glycolate can bother the skin or eyes if someone handles it carelessly. People working around large amounts in factories need good ventilation and gloves. It evaporates at room temperature, so breathing in vapors now and then might cause a headache, sore throat, or light dizziness. Keeping the material locked up and labeling it clearly helps workers avoid accidents. Most reputable companies offer safety training, and guidelines from the Occupational Safety and Health Administration spell out what to do if spills or exposures happen.
I’ve seen community groups grow concerned about chemicals in wastewater. Butyl glycolate breaks down in water over several days, but it doesn’t stick around in the environment as long as some harsh solvents. Careless dumping still leaves a mark, so proper waste disposal keeps both wildlife and nearby families safe. More cities push manufacturers to use just enough to get the job done and recycle when they can. The Environmental Protection Agency lists butyl glycolate as a substance to keep an eye on, and researchers continue looking for greener options that carry less risk without losing cleaning strength.
Consumers at home rarely come across plain butyl glycolate, but diluted forms make it into soaps and sprays. Labels often list chemical names no one can pronounce, but learning to look for glycolates helps people decide how much chemical they want in their homes. Buying products from companies that test for safety and publish their results signals a step toward accountability. Workers in manufacturing plants say real protection comes not only from wearing the right gear, but also from managers who listen if someone spots an unsafe practice.
More research and open conversation about product safety will shape the future use of chemicals like butyl glycolate. Supporting companies with good environmental track records and demanding transparency gives everyone a say in the substances released into homes and communities. Solutions often come from both sides: chemists who invent safer recipes, and ordinary people reading labels and voting with their wallets.
Butyl Glycolate comes up in a fair number of paint shops, cleaning product warehouses, and specialty chemical labs. Many folks might gloss over storage instructions, thinking every solvent lands in the same bin. I’ve watched people think a plastic drum on any shelf will do, only to scramble when leaks or fumes cause headaches or equipment corrosion. There’s more at stake than product loss; mishandled storage can trigger health problems or even lead to regulatory fines. The way a place stores chemicals like this will tell you right away how much they care about both people and long-term operations.
Butyl Glycolate acts as a solvent and an ester but also carries flammability, vapor emission, and skin irritation risks. The chemical evaporates without much warning, and that vapor will creep through leaky seals, broken caps, or poorly fitted containers. Accidents usually don’t start with dramatic spills—in my experience, it’s the slow release that gets missed until a strong odor creeps into the workspace or paint cans rust faster than usual.
Real protection starts with choosing the right container. Don’t cut corners: only metal canisters with tight-fitting lids or thick-walled plastic jugs built to handle organic solvents really work. Thin plastics dissolve over time or crack in moderate heat, and it doesn’t take much for a pinhole leak to start. Label everything with plain language, clear hazard symbols, and a date, then run weekly checks for swelling, discoloration, or sweating around gaskets. It’s surprising how small lapses here quickly turn into major cleanups.
The room or cabinet where you park containers sets the stage for safety. Ventilation keeps vapor buildup low. Keep the chemical stored below 25°C, as warmer spaces increase evaporation and can give way to dangerous concentrations in the air. Lighting should be bright enough to spot leaks fast without being so hot that it adds heat stress. Pouring or mixing batches needs to happen away from open flame, exposed wiring, or electrical boxes. In older facilities, I’ve seen storage spots right near water heaters or breakroom microwaves—move chemicals away from any potential ignition source for peace of mind and regulatory compliance.
I once visited a paint recycling business where operators mixed leftover batches using used beverage jugs and stacked them near a sunlit window. By the end of summer, vapor pressure popped lids and dripped solvent through cardboard boxes, and several workers complained of headaches. Insurance claims nearly sank the business. After that, every new hire got one message: short-term convenience rarely beats the cost of doing things by the book.
Training remains the most effective shield against mishaps. I always recommend hands-on demonstrations covering spills and emergency ventilation because written rules don’t stick the same way. Those who run labs and warehouses should schedule regular drills and use straightforward guides so everyone feels confident responding to leaks or exposure. Provide proper gloves and goggles right near the storage area, not locked in a separate office. Investing in a small flammable storage cabinet costs less than recovering from one serious accident, and businesses show employees that their safety takes priority. Culture changes when safety isn’t just a policy on paper but something everyone expects from each other, every day.
Butyl glycolate comes up in more places than many folks realize. Factories use it for cleaning, laboratories lean on it for chemical synthesis, and some products even rely on it for fresh scents. This chemical, part of the glycolate family, brings a mix of performance and risk to the table. I’ve handled substances like this in a work shop, and the label warnings always made an impression: eye protection, gloves, plenty of ventilation. That’s not by accident.
Direct exposure tells people plenty about chemical safety. Butyl glycolate, like related solvents, gives off a sharp, noticeable smell. Get a whiff too close, and you’ll feel your nose burn. Allow it to sit on your skin, especially in concentrated form, and irritation follows—redness, itching, sometimes swelling. One peer at a chemical plant had a splash hit his hands. He washed up quickly but felt stinging for a solid hour. Short-term symptoms hit fast: sore eyes, scratchy throat, headaches, nausea. These signals warn the body to back away.
Long-term exposure concerns doctors more. Prolonged contact has the potential to worsen respiratory issues, aggravate skin conditions, and—in animal studies—even affect organs like the liver. Reliable sources from the Environmental Protection Agency and the Centers for Disease Control and Prevention back up these risks. Workers deal with chemicals daily, so safety agencies track precisely how these substances behave inside the body. While no massive human studies have proven chronic toxicity, the evidence is strong enough that strict exposure limits stay in place worldwide.
No substance interacts the same way with every person. Some folks have skin that shrugs off minor irritation, while others might see their conditions flare up fast. Spills at home, thankfully, happen less often. Stores don’t offer butyl glycolate off the shelf, but workplaces—paint factories, labs, cleaning contractors—report accidental releases every year. Details from Material Safety Data Sheets show acute oral toxicity at moderate levels. That means swallowing it can send someone to the ER, but it takes a decent dose.
Vapor inhalation leads to fast-acting headaches and dizziness. I learned from a peer who ignored proper ventilation during routine lab work. He ended up dizzy and nauseated after 20 minutes. Later, he admitted he cut corners on the fan because it felt like overkill. The lesson stays with him years later.
Solutions start with clear awareness. Workers need real gloves—nitrile tends to hold up best—not thin plastic. Goggles matter more than many realize. In my own experience, one drip behind standard glasses can become a trip to the medical tent. Companies should also cycle in fresh air to keep fumes from building up, especially in tight areas.
Spills call for quick action: absorb with nonflammable material, bag up the waste securely, and scrub the spot down with lots of water. Emergency wash stations prove their worth often. Employees, even old-timers, benefit from regular reminders and drills.
Some sectors now check for safer substitutes like water-based cleaners or less aggressive solvents. New guidelines come out every year, driven by stricter regulation and growing worker complaints. Regulatory agencies visit sites more often, and heavy fines push companies to swap old chemicals for safer ones.
Staying safe with chemicals like butyl glycolate isn’t just about reading labels. Personal stories prove that nothing replaces the right equipment, strict routines, and a culture where every question is valid. People learn fast that comfort with a routine shouldn’t become complacency in a hazardous workplace.
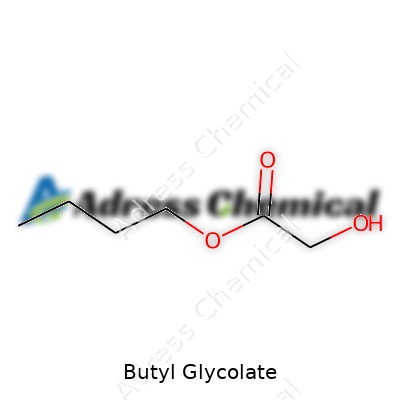
| Names | |
| Preferred IUPAC name | butyl 2-hydroxyacetate |
| Other names |
Butyl 2-hydroxyacetate Butyl glycolate Glycolic acid butyl ester 2-Hydroxyacetic acid butyl ester Butyl hydroxyacetate |
| Pronunciation | /ˈbjuːtɪl ɡlaɪˈkəʊleɪt/ |
| Identifiers | |
| CAS Number | 7397-62-8 |
| Beilstein Reference | 542124 |
| ChEBI | CHEBI:31376 |
| ChEMBL | CHEMBL1692264 |
| ChemSpider | 21810444 |
| DrugBank | DB14096 |
| ECHA InfoCard | 14e6b824-4be5-4d3d-875f-96bcccb52c70 |
| EC Number | 203-316-5 |
| Gmelin Reference | 8228 |
| KEGG | C19364 |
| MeSH | D017718 |
| PubChem CID | 8187 |
| RTECS number | KX4825000 |
| UNII | W7BC8T75Q2 |
| UN number | UN2529 |
| Properties | |
| Chemical formula | C6H12O3 |
| Molar mass | 162.20 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild, fruity |
| Density | 0.954 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.83 |
| Vapor pressure | 0.13 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 3.83 |
| Magnetic susceptibility (χ) | -6.41 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.414 |
| Viscosity | 1.3 mPa·s (25 °C) |
| Dipole moment | 2.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 271.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3847.7 kJ/mol |
| Pharmacology | |
| ATC code | V09CX03 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 72°C (closed cup) |
| Autoignition temperature | 238 °C |
| Explosive limits | Explosive limits: 1.5% - 10.7% |
| Lethal dose or concentration | LD50 oral rat 1728 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1,800 mg/kg |
| NIOSH | KJ9275000 |
| PEL (Permissible) | PEL (Permissible) for Butyl Glycolate: 25 ppm (120 mg/m³) as an 8-hour TWA (OSHA) |
| REL (Recommended) | 2.5 mg/m³ |
| IDLH (Immediate danger) | IDLH: 300 ppm |
| Related compounds | |
| Related compounds |
Butyl acetate Ethylene glycol Ethylene glycol monoethyl ether Glycolic acid Butyl lactate |