Long before most of us cared about esters and their scents, early chemists stumbled upon amyl propionate in their quest to understand how to turn basic alcohols and acids into something new. This ester gained ground during the rise of synthetic fragrances in the late 1800s, joining the cast of chemicals added to perfumes and food. By the time I set foot in a university chemistry lab, professors would mention this compound during lectures about the transformation of raw materials into everyday goods. The industrial development of amyl propionate picked up speed over the twentieth century, driven by demand for food flavorings and solvent applications. Companies tightened processes to improve purity, pushing away from unpredictable yields of old bench-top reactions. In the grand picture, amyl propionate hasn’t set the world on fire, but it turns up in quiet corners of industry where its practical benefits stand out.
Amyl propionate belongs to the ester family, packing a fruity, slightly banana-like smell that has practical uses in flavors and scents. It stands as a colorless, oily liquid, and if you’ve ever worked in a flavor lab, those notes hit your nose immediately. Manufacturers see it as a specialty ingredient, and its balance of volatility and solvency lines up well for cosmetic and industrial applications. Oddly enough, I first spotted it as a minor ingredient in a cleaning solvent—years later, I caught the same hint in a bakery’s strawberry cream filling. Where large volumes of ethyl acetate or butyl acetate can overpower, amyl propionate does its job quietly, making it a favorite for more subtle effects.
This ester boils at around 145°C, which helps with controlled evaporation in fragrance release and even surface coating. Its molecular structure—C8H16O2—pairs a five-carbon amyl group with a propionate backbone. With a refractive index between 1.406–1.410 and a specific gravity of roughly 0.87, the liquid flows easily in most lab setups. The faint, pleasant aroma stands out from the sometimes harsh smell of similar chain esters. It’s practically insoluble in water, though it mixes well with alcohols and organic solvents. Experience teaches that it doesn’t stand up to the most aggressive oxidizers, but it handles day-to-day lab work without fuss.
You run across amyl propionate in technical bulletins labeled as “ISO-Amyl Propionate” or “Pentyl Propionate,” depending on the alcohol used. Drum labels reflect purity of 98% or higher, plus trace details on water, acidity, and color—important for regulatory compliance and process repeatability. In safety data sheets, hazard codes appear mostly for flammability. Factories ship it in steel drums or plastic-liner containers, since glass won’t handle rough transit. The most important reminder on the packaging: volatile vapor risks, and requirements for handling in well-ventilated areas.
Inside the plant, amyl propionate forms through an acid-catalyzed esterification: amyl alcohol meets propionic acid, tossed together under heat in the presence of a strong acid like sulfuric acid. The process chases water out, shifting the reaction toward ester. It sounds simple, but keeping the temperature just right and removing water by-products demands real skill, especially in scale-up. After the reaction, distillation delivers material pure enough for food or fragrance work. Some newer methods turn to enzyme catalysis for greener processes—though big factories stick to classic synthesis, given proven reliability and less process complexity.
Amyl propionate resists breakdown in mild conditions, but reacts with strong acids, bases, or oxidizers. In lab practice, saponification with sodium hydroxide yields amyl alcohol and sodium propionate—reversible with thought-out planning. Product developers sometimes tweak the alcohol portion for specific properties, trading amyl for isoamyl or hexyl chains for a slightly different scent or evaporation rate. In coatings, crosslinkers anchor the ester on polymer chains, adding toughness or impact resistance. Each reaction pathway creates options for formulators to fine-tune blends.
Chemical catalogs list amyl propionate under names such as pentyl propionate, n-amyl propionate, isoamyl propionate, and 1-pentyl propionate. Fragrance and flavor firms sometimes code it as FEMA 2935 or EINECS 205-071-3. It’s easy for a newcomer to trip up over the variety of trade and technical names, but careful label reading and cross-checking with CAS number 624-54-4 clears up confusion fast.
Factories enforce no-nonsense controls for storage and handling. Amyl propionate ignites under spark or flame, needing cool, well-ventilated storage. Workers use splash-proof goggles and nitrile gloves for sampling or blending. Spills call for quick clean-up with absorbent pads, and fire crews keep foam extinguishers close by. Local workplace safety rules spell out air monitoring requirements and exposure limits, usually modeled after broader solvent regulations. Over several years in labs, my colleagues and I developed muscle memory for these safety steps—no one wants to see avoidable incidents popping up on the morning shift.
Firms use amyl propionate as a specialty solvent in coatings and inks, giving good flow and drying behavior without overpowering odor. In perfumes, it delivers a soft green note, blending well in floral or fruity bases. Food scientists use it to add depth to fruit flavors—strawberry, pear, apple—in candies, fillings, and beverages, though in strictly limited doses. Its low toxicity and volatility make it useful for nail lacquers and some personal care products. Industrial chemists blend it with other esters to make cleaning fluids for electronics or delicate machinery. My years in product development taught that small tweaks with esters like this can rescue a formula stuck between poor stability and off-odors.
Chemists continue searching for greener, more energy-efficient synthesis methods, making enzyme catalysis and recyclable solvents regular topics at industry conferences. Flavorists experiment with blends to target new regional taste preferences—especially in Asia-Pacific and South America—where exotic fruit and sweet profiles fuel innovation. Industry partnerships bring together food, fragrance, and materials teams, creating unexpected roles for amyl propionate in biodegradable plastics and low-VOC coatings. As regulatory demands for clean-label and sustainable sourcing ramp up, research labs pour resources into tracking trace impurities and improving environmental impact.
Animal studies rate amyl propionate as relatively low in acute toxicity, with lethal dose thresholds well above routine exposure. Nonetheless, repeated skin contact can dry or irritate sensitive skin, and inhalation of vapor in unventilated rooms will spark headaches or nausea after prolonged exposure. The compound shows low bioaccumulation, breaking down quickly in air or soil. Scientists look for possible metabolic byproducts, but so far, assessments by environmental agencies haven’t flagged major chronic risks from controlled use. In my own practice, strict PPE and adherence to fume hood protocols have always kept exposures within limits.
Shifts toward sustainable products open new doors for amyl propionate, especially with markets chasing bio-based esters for everything from flavors to specialty coatings. If enzyme-catalyzed production drops in cost, plant-based feedstocks could take over from petrochemical precursors. Regulatory shifts—Ban on high-VOC solvents, new flavoring safety rulings—could push manufacturers to reformulate around ingredients like amyl propionate. The flavor and fragrance sectors keep pushing the boundaries, searching for tiny chemical tweaks that can support cleaner, friendlier labels. Collaboration across research, manufacturing, and regulatory arms will shape whether this quiet ester becomes more central in everyday goods, or stays a specialty ingredient for niche markets.
Amyl propionate doesn’t show up in your average kitchen conversation, but products at home often carry its signature. Walk near a bottle of nail polish remover or open a fresh bottle of perfume and chances are, you’ve caught a whiff of it. Amyl propionate’s fruity scent gets it into the world of fragrances, lending that familiar pear-like lift to both basic body sprays and luxury perfumes. Chemical names often sound intimidating, though, and I see how people worry about what’s inside those long ingredient lists on everyday goods.
The story of amyl propionate comes down to performance and scent. Solvents, for example, make everything from thinners to nail polishes actually usable; without them, you’d scrape chunky paint onto your walls, or drag a goopy mess across your fingernails. Amyl propionate dissolves many different organic substances, which makes it useful for tidy, even application. Manufacturers lean into this because customers expect nail lacquer to dry quickly and stay smooth.
Inside the food world, amyl propionate gets used as a flavoring agent. Bite into a fruit-flavored candy, jelly, or chewing gum and the rounded notes of green apple or pear often owe something to this ester. Food chemists use it in concentrations that deliver taste without crossing into synthetic weirdness. The compound appears on ingredient labels as “artificial flavor,” which sometimes leads to suspicion, but it’s often about sensibly replicating familiar tastes on a large scale.
People have an instinct to be nervous about chemicals with unfamiliar names. That caution makes sense in a world packed with additives, but it’s worth comparing amyl propionate to other solvents in the same class. Unlike some old-school solvents, which set off warning bells for toxicity or flammability, amyl propionate comes across as relatively mild. Still, it shouldn’t be inhaled in large amounts or splashed in the eyes. Regular ventilation and simple safety steps at home, like recapping bottles and avoiding skin contact, help limit risk for anyone using beauty supplies or paint strippers.
Most of us don’t think twice about throwing empty nail polish bottles in the trash, but solvents like amyl propionate should be treated with some extra respect. Local regulations for household hazardous waste make sense here, since improper dumping can mean traces of the solvent end up in rivers or soil. The greatest solution for households: collect used-up solvent containers and drop them off at special collection days. In the bigger picture, industry can lower waste by recycling solvents and sticking with compounds that have a lower environmental footprint.
Transparency in labeling has always mattered to me. Anyone confronted with mysterious chemical names needs more than just a list—they need context in plain language. Companies could step up and share not only what goes into products, but why. Knowing amyl propionate helps polish dry without clumps, or that it brings out that fruity zing in candy, removes some of the nervousness from consumers. Most folks respect honesty, especially when it comes to substances entering their homes or bodies.
Amyl propionate is an ester with a sweet, fruity odor. Manufacturers often go for such ingredients when crafting different flavors and fragrances. This compound, found in cosmetics and sometimes food, behaves like many other esters popular with chemists who want a fruit-inspired aroma that isn’t too harsh or artificial. Anyone who ever sniffed a cherry-flavored lip balm or chewed gum flavored with hints of pear might have already crossed paths with this ingredient without realizing it.
Safety makes its way to the top of the list for anyone mixing or using chemicals near the skin or inside recipes. Amyl propionate has had its turn under the microscope, particularly by organizations like the Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). Both agencies examine compounds for toxicity and allergenic potential before saying okay to broad use. Right now, the FDA lists amyl propionate as generally recognized as safe (GRAS) for use in food. This status falls to compounds with such a long track record of safe use and low levels of toxicity that, unless a person were to ingest grossly abnormal amounts, the body handles it without concern.
Skin absorption sparks questions, too. Cosmetic formulators rarely use pure, undiluted amyl propionate in high doses. Small concentrations—usually less than one percent—get mixed with other ingredients. So far, cases of irritation or allergic reactions appear rare in the published studies.
One fact worth noting: repeated, direct exposure to high concentration vapors from the chemical may cause irritation, just like a strong perfume might sting sensitive skin or eyes. This risk doesn’t show up in typical product use, so most shoppers have little to worry about. Recent animal studies haven’t turned up much evidence of cancer, birth defects, or gene-level harm, so the compound has a relatively clean bill of health, especially compared with some older perfume and solvent chemicals now phased out.
Still, no one should take a free-for-all approach with ingredients, no matter how mild they seem on paper. Some people with especially sensitive skin can react to things most folks ignore. Labeling practices that include potential irritants help people avoid nasty surprises. Anyone prone to allergies or rashes learns fast to scan ingredient lists with an eagle eye, not just for amyl propionate, but for anything that caused trouble before.
Personal stories and science both have a place here. I remember my time working with food ingredients in quality assurance, and it became clear that trace details matter. Reliable manufacturers run their ingredients through rigorous quality control, and respected brands usually provide safety data sheets on demand. Smaller brands and overseas online sellers can sometimes play fast and loose with standards. I’d stick with products with transparent labeling and avoid gray-market options if you want fewer surprises.
For a consumer, the safest route comes down to testing before wide use. Try out new cosmetics on a small patch of skin, and keep an eye out for any signs of redness or discomfort. This same caution works for trying new treats containing unfamiliar flavors.
Ongoing research helps spot long-term effects early. Watchdog organizations sometimes add new caution notices as evidence rolls in, so keeping an eye on updates from the FDA or EFSA helps too. For most people, amyl propionate in modern concentrations doesn’t set off alarm bells. Simple precautions and solid information turn this once-unknown chemical into just another harmless part of daily routines.
Anyone who’s worked in a lab or fussed about ingredients in perfume or flavoring knows the importance of clarity in naming things. Chemistry, with its endless abbreviations, can make your head spin reading through lists of names. "Amyl Propionate" pops up in industries like fragrances, food flavorings, and even solvents. But what does it actually mean on a practical level for anyone who cares about chemistry, production, or even health?
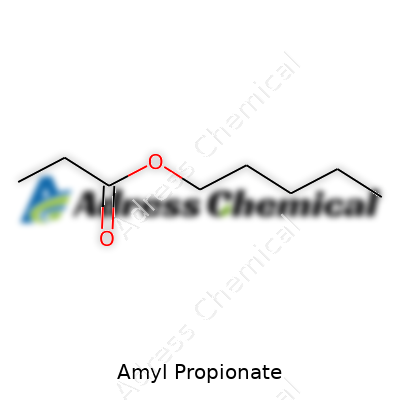
Amyl Propionate’s formula—C8H16O2—might look like another jumble, but knowing what’s behind it tells a bigger story. This isn’t just a code. It's a direct map to how the molecule behaves and where it fits in the wider world of esters. Five carbons in the amyl group, three carbons in the propionate piece, two oxygens for that familiar ester bridge. Toss a bunch of hydrogens in to complete the structure and that’s how you get C8H16O2.
I’ve handled bulk chemicals in manufacturing, and there’s a difference between knowing a product is “fruit-scented” versus knowing it’s amyl propionate with C8H16O2. That chemical formula tells you what kind of reactions to expect, what breaks down if the stuff sits in the sun, and how it’ll behave in blends. People fuss over safety sheets for a reason: knowing what's in a bottle helps folks stay safe and troubleshoot problems during production.
Chemical formulas also play a huge role in product labeling. Regulations don’t just ask for trade names; they want the chemistry spelled out for compliance and transparency. People with allergies, concerns about environmental impact, or businesses that depend on eco-certification, all need clear formulas like C8H16O2 spelled out. There's no hiding behind brand names or generic labels anymore.
A simple ester like amyl propionate shows up in more than test tubes. Perfume makers love the fresh, juicy, almost apple-like aroma. Food chemists lean on it for its sweet flavor kick. Folks with a hand in sustainable chemistry notice that its clean breakdown products reduce long-term residue. Properties like volatility and solvency aren’t wild guesses—they tie directly to C8H16O2.
Misinformation crops up when people throw around generic names. Calling something "amyl propionate" without a formula can lead to mix-ups with different isomers or blends. Chemistry isn’t just a matter of taste or small differences. A missed atom or wrong arrangement changes how a product acts, affecting everything from aroma to safety profile.
In my experience, clear labeling and honest discussion round out better solutions for everyone. Nobody likes surprises in their supply chain, least of all with complex substances. Encouraging suppliers and manufacturers to use precise chemical formulas cuts back on mistakes and builds trust. Schools, too, gain from teaching students that formulas are more than homework—they’re a practical tool everywhere from the laboratory to the marketplace.
For anyone navigating ingredients, whether by necessity or curiosity, understanding the chemical formula behind something like amyl propionate pays dividends. C8H16O2 isn’t just science—it’s information for better decision-making, improved safety, smoother production, and transparency for every stakeholder.
The first time I came across a bottle of Amyl Propionate in the lab, I asked my supervisor if it was really as fussy as the label made it sound. He gave me the look—you know, the one that says “you spill it, you deal with the fire.” That’s how quickly this stuff can flip from handy solvent to safety nightmare when storage goes wrong. Amyl Propionate sits among those chemicals that don’t offer much room for error.
Storing Amyl Propionate isn’t a task for folks who ignore faded warning stickers. Start with temperature: room temp feels simplest, but any spot that ends up warmer than 30°C promises trouble, especially if you’ve got sunlight pouring in. A hot storage area speeds up degradation, sometimes causing pressure to build in the container. I’ve seen closets with direct window sun turn into slow-brewing disaster zones.
This compound carries a fruity, pleasant odor—until it escapes and makes the room smell like cheap candy and headaches. Leaky caps or overused plastic lids only ask for trouble. Glass works best, though high-quality metal can step in if it won’t corrode. Ensure that cap fits snug, check gaskets for splits, and never put old solvent in something you can’t seal up tight. You won’t just keep your storage room clean; you’ll block evaporation and slow down breakdown.
Water doesn’t mix well with many esters, and Amyl Propionate stands as proof. Humid conditions let water seep in and cause slow chemical changes. Stash bottles somewhere dry, on shelves above floor level—not in a damp basement or poky corner near a sink. If you’ve walked into a supply closet and noticed a musty smell, rethink that spot for anything sensitive.
You don’t want Amyl Propionate anywhere near strong acids, alkalis, or oxidizers. I learned this one quick after watching a spill near a bleach bottle go from “oops” to “evacuate the hallway.” Treat storage like setting up seats at a family reunion—some folks just shouldn’t sit together. Use plastic bins with dividers or clear labels to stop cross-reactions. A fireproof cabinet adds real peace of mind, especially in places that see a lot of traffic or get forgotten over the holidays.
A locked cabinet with a tight seal keeps unwanted hands away, but air should circulate enough to keep fumes from building up. Over the years, I’ve seen too many old storerooms with one vent, low airflow, and the unmistakable tang of old solvents in the air. That’s not safety, that’s just waiting for a problem.
Grab a Sharpie and mark the date on that bottle label the day it arrives—not just for fun, but so nothing lurks forgotten on the bottom shelf past its prime. Overripe solvent sometimes means more risk than reward. Routinely check the stock, use up the oldest first, and follow up with proper disposal for any bottle that looks off-color or clumpy.
Amyl Propionate doesn’t care for cutting corners. Safe storage demands steady temperature, dryness, tight seals, careful separation, and occasional housekeeping. It sounds like extra work, but nothing ruins a good lab day like an emergency caused by a forgotten or badly stored solvent.
You stumble across the name Amyl Propionate, maybe in a list of flavoring ingredients or buried in the technical sheet for a perfume. The word sounds pretty harmless. It brings to mind fruit or something that gives off a pleasant whiff. In practice, this compound pops up in flavoring, fragrances, and as a solvent. If you work in a lab, or someone stacks products on your warehouse shelves all afternoon, a brush with this chemical feels almost routine.
Amyl Propionate mixes two things: amyl alcohol and propionic acid. That blend produces a substance with a sweet, pear-like scent. It’s a favorite where folks seek fruity notes, whether in candy flavorings or body sprays. Looking at the safety data for it, though, I spot a few red flags that don’t always get the attention they deserve.
Getting this chemical on your skin usually leads to light irritation. Small exposures—an accidental touch or a whiff—won’t send someone rushing to the emergency room. Extended contact, though, can start a rash. Inhaling it causes throat irritation or a burning nose if the vapors get too heavy in a closed room. Weak air flow in work zones, or storage mishaps, sometimes make minor situations turn ugly. Folks with asthma or allergies might cough or have trouble breathing after a spill.
Big spills on factory floors aren’t a common sight, but broken containers in storage or leaky pipes make things slippery fast. Amyl Propionate is flammable, and the vapors can catch fire more quickly than many expect. Temperature spikes—a summer day in a delivery van, or equipment running hot—make fumes heavier. I remember the panic in a warehouse after a leaking bottle nearly touched a sparking outlet. No fire started, thankfully, but the lesson stuck. Firefighters already treat this chemical like something that can flare up just as easily as gasoline.
People lose track of how work routines creep into the home. Just because a chemical has a fruity smell and shows up in flavors doesn’t mean it should float around the house. Gloves and goggles seem basic—yet someone skips them because “it’s not formaldehyde.” Tight lids, well-marked containers, and decent ventilation block most of the risks. Nobody needs a fume hood for a single bottle in the shop, but propping open a window or running a fan never hurts.
Frequent news about chemical accidents often involves the same set of mistakes: missing warning labels, ignored safety sheets, or overconfidence built on familiarity. A detailed label and a few lines in the training manual cut down the odds of trouble. Images of bandaged hands and coughing staff in an emergency room really bring this home. Even the pros slip up. Regulations by groups like OSHA might seem strict, but one serious burn tells you why they exist.
Simple steps work best. Teach the real risks—not just the rare horror stories. Get gear that sits close and feels easy to use. Make sure everyone knows the nearest sink and eye wash. Imagine what happens if the fumes collect and someone lights a cigarette at the wrong moment. Regular staff reminders, clear labeling, and honest conversation keep small risks from turning into full-scale emergencies.
Amyl Propionate brings comfort through smell and taste, but it demands due respect. Skipping precautions is a short walk to regret—no matter how gentle a chemical seems on the surface. Practical safeguards, workplace training, and a dash of healthy respect offer the surest path around potential hazards. Every so often I remember the lesson: a pleasant scent is no excuse for cutting corners with safety.
Amyl propionate rarely gets a mention in everyday conversation. Most people probably haven’t seen its name on a label. Yet, walk down the cleaning aisle or browse perfumes at a department store, and you’ll run into its handiwork. This clear liquid slips into formulas to give products a subtle, fruity scent that feels familiar but hard to pin down. I remember the first time I noticed a sweet, pear-like undertone in my favorite air freshener. That experienced nose might pick up amyl propionate, lending that smooth, fresh smell that doesn’t smack of anything artificial or overpowering.
In the flavor world, manufacturers keep looking for that balance between sweet, tangy, and natural. Amyl propionate plays a role here too. Bakers and confectioners use it to create flavor profiles for candy and baked goods. Instead of a bold artificial note, it offers a background taste that rounds out fruit flavors, especially apple and pear. From what I’ve seen in food science, blending flavors with small helpers like this compound makes the end product taste closer to the real fruit—even if the fruit never touches the recipe. It cuts through the harsh edges of other additives and brings gentle sweetness to candies, gum, and even ice cream.
Cosmetic creators turn to amyl propionate for its power as a solvent. In nail polish, it helps dissolve other ingredients and allows formulas to spread smoothly on the nail. This cuts drying time, so polish doesn’t smudge while you fumble for your keys. Its mild scent improves the polish experience for folks sensitive to chemical odors. Not just for nails, it finds its way into some perfumes and lotions, where its pleasant aroma and good solvency make it a better alternative to harsher chemicals.
The paint world values amyl propionate for many of the same reasons. Manufacturers mix it in certain lacquers and inks—not just for scent, but for its ability to pair well with other ingredients and promote a smoother application. That combination matters in workshops where time and finish both count. Safety matters here, too. Though amyl propionate generally rates as having low toxicity, inhaling concentrated fumes can irritate the nose and eyes. Proper ventilation and handling go a long way in paint shops and labs. Having worked around solvents, I know good safety habits become second nature, but education and enforcement still warrant attention.
Some push for greener chemistry want cleaner, less volatile chemicals in everyday goods. Amyl propionate already counts as a relatively gentle solvent, but the industry keeps searching for options that break down faster in the environment and don’t linger as pollutants. Shifting to renewable sources for raw materials and refining production methods cuts down the chemical’s environmental footprint. In the meantime, reading up on product labels at home helps anyone learn what goes into daily items, reminding us that even a humble, behind-the-scenes ingredient like amyl propionate shapes our experience.
Plenty of chemical names can ring alarm bells, but most folks only notice them glued to the small print on a lotion or foundation bottle. Amyl propionate, for most, feels like one of those mystery ingredients. Basically, it’s an ester made from amyl alcohol and propionic acid, used for fragrances and as a lightweight solvent in all sorts of beauty concoctions. Flip through ingredient lists on perfumes, skin creams, or hair sprays, and it’s easy to spot how companies turn to this compound for its signature fruity scent and ability to help formulas feel smooth.
Straight talk — the world of cosmetic safety is a maze. Some countries police ingredients tightly, others less so. Amyl propionate has already made the rounds in labs. Groups like the Cosmetic Ingredient Review (CIR) in the United States and the European Scientific Committee on Consumer Safety (SCCS) have dug through the numbers. Most studies point to amyl propionate being low-risk for skin and eyes, especially in the tiny amounts added to scented products or creams.
Still, safety doesn’t just hang on one study. Years back, reports showed contact with high concentrations could sting eyes or irritate skin. That’s easy to sidestep — cosmetic formulas rarely include anything at unreasonably high doses. Most research keeps circling around how amyl propionate works in the dilute mixtures found in real-off-the-shelf cosmetics. So far, no widespread allergy scares, no signs it builds up in the body, no strong evidence it could trigger genetic changes, birth defects, or the big C.
Reading the word “safe” builds no comfort without real monitoring behind it. People have a right to expect the balm or mist they swipe on won’t turn into tomorrow’s news headline for the wrong reason. Too often, beauty product makers chase new scents or textures to keep buyers hooked, then lean on decades-old rules or weak testing to greenlight a formula. What keeps safety solid isn’t a label, it’s a chain of checking, retesting, and public reporting that works for today’s world, not just the last generation’s habits.
There’s a trap in letting small, non-scary risks add up. A dab here, a spritz there, and the cocktail of daily exposures can wind up bigger than anyone realizes. Skin varies, too. A formula that sits fine on me might irritate someone else. Much of my own approach comes down to patch testing, nose work (allergic to too much fake pear), and making sense of the logic behind every ingredient. Amyl propionate, so far, hasn’t ticked any of my personal “avoid” boxes, but that doesn’t mean I don’t keep an eye on big reviews or updated warnings.
Making this whole picture safer calls for both watchdogs and good habits. Pressure from everyday buyers goes a long way. Seek out brands with transparent ingredient lists, clear testing info, and honest answers to safety questions. It’s possible to keep using both tradition and science — not just what’s easy to market. Regulators can force more frequent updates, push for full ingredient disclosure, and roll out bigger post-market surveillance, not just pre-market tests. For the allergy-prone or cautious, patch testing and sticking with trusted brands makes sense, but the load to keep cosmetics safe should land mostly with the product makers and watchdogs, not just overloaded customers combing through Latin ingredient names.
Through all the noise, amyl propionate still wears its “safe enough for now” label in most cases, but a culture that stops at “safe enough” won’t always spot early warning signs. Keeping trust in the beauty aisle or online checkout box takes open data, steady review, and less hiding behind scientific jargon.
Amyl propionate stands out mostly because of what you notice first—its scent. This liquid gives off a fruity, pear-like aroma that feels stronger than what you’d expect from a simple bottle on the lab shelf. Used in perfumes and flavors, it doesn’t just smell good. It tells a bigger story about how people add character to everyday products. I remember working in a small soap shop, and that sharp, juicy whiff from just a drop of amyl propionate changed a bland batch into something customers would pick up just to breathe it in.
On a physical level, amyl propionate acts more like a clear-minded spirit than a heavy syrup. It pours as a clear, colorless liquid with a slightly oily feel but it avoids sticking to your fingers the way some heavier esters do. Its boiling point sits around 146 degrees Celsius, which puts it in a spot where it won’t just evaporate at room temperature, but can still be coaxed into the air by a warm hand or the back of your neck. The density drops in lighter than water—about 0.87 g/cm3. Toss a drop in a glass of water and you’ll spot it floating up top, refusing to mix in.
Switch to the chemistry lab and, at its heart, amyl propionate belongs to the ester family. In practice, this means resistance to dissolving in water, but it will dissolve easily in alcohols and most organic solvents. Mixing into oil-based solutions, paints, and other perfumes feels natural to this compound. The backbone of amyl propionate features a propionic acid joined with amyl alcohol. These two together form its classic fruity punch.
This substance doesn't ask for trouble: it rarely reacts with other chemicals unless pushed hard. Strong acids or bases can break it apart, sending it back to its parent acid and alcohol, but for day-to-day handling in a lab or factory, it stays stable. Its vapor, though, catches light fairly easily, with a flash point around 39°C. Anyone working with this liquid needs to stay aware—enough vapor in a closed space and one stray spark can set off a fire. Most stories in chemical safety classes warn about solvents like this, but they feel real when you’re cleaning up a spill and realize those fumes you’re breathing can do more than just make you lightheaded.
In high school chemistry, no one ever talked about how the chemicals that brought a whiff of green apple to a candy, or a soft note to a lotion, had to walk a fine line. They deliver scent, flavor, and smooth texture, but only as long as they behave themselves with other ingredients. Amyl propionate is proof that small shifts in a chemical's makeup change everything—it’s safe in perfume but not something you’d let linger around sparks or flames.
So the lesson for people handling compounds like this isn’t just to follow the safety sheets or memorize physical values. It’s about understanding how that sharp sweet scent, that floats out with a light oiliness, brings a lot of value to everyday items while asking for real care and respect in handling. Suppliers and makers need good ventilation, storage away from heat or ignition, and the right containers because amyl propionate’s properties draw both possibilities and hazards into the room.
With materials like amyl propionate, basic habits build the biggest difference. Staying aware of flammability, using proper storage, and never underestimating a fruity smell in the workshop or studio—these are the steps that keep accidents rare and allow this small, fragrant liquid to add its charm without surprise. Talking about esters in plain language and sharing stories from real workplaces can teach far more than graphs and charts ever do.
Amyl propionate lives on a long list of chemicals that seem harmless at first glance. It smells a bit like fruit, pops up in fragrances, and even tricks some noses into thinking of pears. But no matter how sweet it smells, it brings its own set of hazards. Living with chemicals for years, anyone picking up a bottle in a lab or warehouse knows: the storage story matters more than the label’s promise of aroma.
I learned early on that solvents with a low flash point ask for respect. Amyl propionate doesn’t need high heat to turn flammable — even a regular room on a hot day could spell trouble. It never pays to take that kind of risk lightly. Letting containers sit near equipment that gives off sparks or heat, even if unintentional, can turn a smooth day into a disaster.
Putting it in a cool spot changes the whole calculation. Choose a section of the storage area away from sunlight, far from radiators or heat vents. Heavy-duty shelving, not rickety wooden ones, keeps everything steady. From experience, bottles sitting on unstable racks don’t always stay upright either. That turns minor tremors into possible spills.
Humidity also doesn’t go ignored. Leaky pipes overhead or a muggy room could threaten the integrity of containers. Amyl propionate will break down faster or eat through a poorly sealed cap if moisture gets in. I always check seals, replace any that feel flimsy, and mark containers with dates using a thick marker. If a cap turns sticky or doesn’t tighten right, it’s time for a replacement — not an “it should be fine” moment.
Regular ventilation helps lower risks too. Solvent fumes, left to build up, aren’t just unpleasant — they can knock someone out or create a dangerous atmosphere. Even a cracked window or exhaust fan, modest as it sounds, keeps the air moving. In my own work, airing out a storage room has solved plenty of headaches, both literal and regulatory.
Mixing amyl propionate with strong acids, oxidizers, or bases creates hazards faster than most folks expect. Too many people I’ve met treat chemical compatibility as an afterthought. Storing this compound far from bleach, nitric acid, or even peroxide keeps nasty surprises at bay. I keep an up-to-date list by the door as a reminder of which chemicals stay separated.
Locking doors, not just closed cabinets, keeps wandering hands — especially kids or visitors — away. In every facility I’ve worked, busy times sometimes tempt workers to ignore this step. A padlock or combination lock offers peace of mind that signs on the door never quite provide. Simple protocols beat clever signs every time.
No matter how careful storage gets, spills happen. Absorbent materials nearby, clearly labeled, make a big difference. Throwing sawdust or sand haphazardly in the corner doesn’t help in a crunch. I’ve found that labeling buckets and keeping clean gloves close makes cleanup fast and efficient. Employees should know exactly where these supplies live—not search for them during a spill.
Fire extinguishers, just a step away, shouldn’t just be for show. Having a Class B rated unit close by, with an inspection tag in the last month, keeps everyone ready for those rare emergencies. I test alarms monthly rather than trusting they’ll work in the rush of an incident.
Safe storage of amyl propionate doesn’t demand expensive equipment. It asks for straightforward habits and a clear-eyed understanding of how chemicals behave when left alone or treated carelessly. Keeping a written checklist, providing basic training, and changing out old containers as soon as they show wear stays at the forefront of my approach every day on the job.
Amyl propionate doesn’t usually catch headlines, but it pops up in spots like perfumes, nail polish removers, and even flavors for foods. Its apple-fruity aroma makes it a favorite among folks who work with scents. You find it in the background of a lot more than you might expect, and the compound gives cleaners and solvents that sweet kick.
The first thing many people want to know: what happens to amyl propionate once it gets flushed down the drain? Here’s where things get interesting. This compound belongs to a group known as esters. Mother Nature—especially microbes—loves munching on esters. So, in the right conditions, amyl propionate can break down into smaller, less worrisome pieces pretty fast. Most lab tests show this stuff breaks down in water and soil within days. When I read through environmental chemistry reports, the story is usually the same: not much leftover residue after a short period. That gives it a leg up over ingredients that stick around for months or even years, stubbornly turning up in rivers or lakes.
Consumers and companies love slapping “biodegradable” labels on things. But real-world results count more than any label. Biodegradable just means microbes can break it down over time, but it doesn’t always mean safe by default. Some things snap apart quickly but leave toxic leftovers. I’ve seen this with common industrial solvents that start out harmless on paper but wind up hurting fish or plants in tests.
Amyl propionate tends not to hang around in harmful concentrations. Research in environmental science journals backs this up: its breakdown products—propionic acid and amyl alcohol—don’t pile up in water systems. Neither has a reputation as a major troublemaker. Still, just because a chemical is “better” than others doesn’t mean using more is okay. Spills, dumping, or overuse create their own headaches. Anyone who’s walked by a river near an industrial park can smell the results.
Calling something “environmentally friendly” always makes me a bit skeptical. Production still chews up resources, and transportation burns fossil fuels. Factories dumping any chemical into drains, even ones that break down fast, create pressure on wastewater systems. Most water treatment plants count on chemicals breaking down quickly, and amyl propionate usually cooperates. That helps cities meet clean water standards with less fuss. But in places where water systems start to buckle—think aging pipes or packed population centers—even fast-degrading chemicals can build up, at least for a while.
It’s smart to ask: can the world do even better? A lot of progress comes from reducing use in the first place. I’ve seen small manufacturers reformulate products to squeeze out unnecessary chemicals—and save money in the long run. Stronger oversight keeps everyone honest about what actually leaves a plant and how it gets managed. On the consumer side, voting with your wallet works. Buying from companies that show clear data on environmental impacts, not just green slogans, matters.
For folks making products, safer alternatives deserve a close look, even if switching costs more in the short run. Regulators and businesses could pool resources to test safer formulas—not just trust the old ones. The story of amyl propionate looks better than many chemicals on the market, but the push for truly responsible use never stops.
| Names | |
| Preferred IUPAC name | Propyl pentanoate |
| Other names |
Propanoic acid amyl ester n-Amyl propionate Pentyl propionate Propionic acid pentyl ester |
| Pronunciation | /ˈæmɪl proʊˈpiːəneɪt/ |
| Identifiers | |
| CAS Number | 540-42-1 |
| Beilstein Reference | 1209281 |
| ChEBI | CHEBI:88534 |
| ChEMBL | CHEBI:88913 |
| ChemSpider | 14101 |
| DrugBank | DB14012 |
| ECHA InfoCard | echa-info-card-100926700 |
| EC Number | 203-686-1 |
| Gmelin Reference | 152812 |
| KEGG | C19609 |
| MeSH | D02.241.081.211.192 |
| PubChem CID | 8095 |
| RTECS number | AJ8775000 |
| UNII | Y46LV6E7JS |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C8H16O2 |
| Molar mass | 130.19 g/mol |
| Appearance | Colorless liquid with fruity odor |
| Odor | fruity |
| Density | 0.869 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 2.8 |
| Vapor pressure | 0.7 mmHg (20°C) |
| Acidity (pKa) | pKa ≈ 25 (very weak acidity, typical of esters) |
| Basicity (pKb) | pKb: 15.86 |
| Magnetic susceptibility (χ) | -7.31E-6 cm³/mol |
| Refractive index (nD) | 1.406 |
| Viscosity | 2.2 mPa·s |
| Dipole moment | 1.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 324.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -481.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4356.0 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| Flash point | 66 °C |
| Autoignition temperature | 411 °C |
| Explosive limits | Explosive limits: 0.9%–7% |
| Lethal dose or concentration | LD50 oral rat 6,820 mg/kg |
| LD50 (median dose) | LD50 (median dose): 9370 mg/kg (rat, oral) |
| NIOSH | TR8225000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 100% |
| IDLH (Immediate danger) | IDLH: 200 ppm |
| Related compounds | |
| Related compounds |
Amyl acetate Isopropyl propionate Butyl propionate |