Long before paint thinners found their way to home improvement aisles, 2-Methoxy-1-propanol started turning heads in industrial chemistry circles. Back in the mid-20th century, solvents like this helped shift production floors away from bulkier, slower evaporating liquids. Researchers noticed right away that ether-alcohol compounds could lift performance in cleaning formulations and ink making. Companies hunted for alternatives with lower odor and less aggressive profiles. The industry moved forward with years of patent filings and incremental improvements, spurred by regulatory changes and growing demand for more specialized manufacturing aids. Across Europe and the United States, chemical plants scaled up output for paper mills, factories, and electronics shops, each wanting better solvency without excessive volatility. The story of 2-Methoxy-1-propanol highlights the urge to replace old workhorses like toluene and xylene, not out of theoretical neatness but through real pressure at the point of use.
Not many outside coatings chemistry could distinguish 2-Methoxy-1-propanol just by name, but it sits on plenty of shelves as propylene glycol monomethyl ether (PGME). This clear, thin liquid doesn’t turn heads on sight or smell; that understated profile sets it apart from heavy, pungent solvents. Suppliers ship it under names like Dowanol PM or Propasol, targeting industries that want a combination of strong solvency and gentle evaporation. As a glycol ether, it covers a lot of ground — thinning paint, cleaning printing presses, stripping resins, and stretching water-based formulas. PGME stands out for balancing flash-off speed with enough open time to avoid streaks, making painters, carpenters, and press operators quietly grateful. This versatility extends beyond paints into varnishes, inks, cleaning fluids, and even pharmaceutical processes. Technologists lean on 2-Methoxy-1-propanol for daily production runs where consistency and ease of use keep projects on track.
This chemical flows as a colorless liquid, showing a boiling point around 120–125°C and a melting point just below freezing. It carries a mild, slightly ether-like odor, less sharp than most painter’s solvents. Its density rests close to 0.92 g/cm³, and it dissolves into water without fuss, thanks to its ether linkage and hydroxyl group. Unlike heavier solvents, 2-Methoxy-1-propanol flashes off at a steady, moderate pace. A vapor pressure of about 10–14 mmHg (at 20°C) gives operators a dependable evaporation curve. It mixes smoothly with alcohols, ketones, and various hydrocarbons, making it a favorite for blend formulations. For me, that means fewer surprises when trying a new water-based finish or running a test batch of ink at the press. Chemical stability remains high under normal conditions, allowing storage and transport without constant worry about decomposition. Flammability does raise concerns above 30°C, so storage needs proper ventilation and grounding, but it behaves with more predictability than many older hydrocarbon mixtures.
Product labels offer a dense cluster of numbers and signal words — purity levels usually hit above 98%, and water content stays low to avoid unwanted hazing in surface coatings. CAS number 107-98-2 marks compatibility databases, and proper UN codes appear for shipping. Barcode scanners pull up everything from batch number to flash point. Hazard pictograms include flame and exclamation marks, reminding workers not to let their guard down. Labeling requirements under the Globally Harmonized System set out risk phrases relating to eye and skin irritation risks. Proper storage temperature and exposure limits help keep workplaces organized and safe. There’s a lot more to these labels than legal compliance; clear details give purchasing teams confidence and allow manufacturing teams to spot any deviation in what arrives from the supplier. When I see accurate specs and safety data, I know the production process won’t grind to a halt from mystery side reactions or unexpected residues.
Production runs for 2-Methoxy-1-propanol typically start with the reaction between propylene oxide and methanol, kicking off in the presence of a basic catalyst. This approach favors simplicity, steering clear of complex intermediates. In practice, temperature and pressure control remain vital; factories marry batch size to the heat transfer capacity of reactors to sidestep runaway exotherms. Post-reaction phases include distillation and drying, stripping out unreacted methanol and light ethers, refining the product’s purity well beyond what’s needed for most consumer chemicals. Mixing experience with good instrumentation, chemists optimize every variable, watching for shifts in byproduct loads or slightly off-ratio feedstocks. Environmental controls help trap fugitive emissions from the process line, with scrubbers and condensers preventing glycol ethers from escaping into the local air. Scaled batches feed directly into drums and ISO tanks, ready for transport to downstream blenders or direct end users.
2-Methoxy-1-propanol’s open chain and functional groups make it a good candidate for mild chemical transformations. The hydroxyl group allows conversion to esters, supporting a range of specialty coatings and lubricant bases. Modifications through etherification or acylation extend its use into surfactant synthesis and plasticizer preparations. As a solvent and a reagent, it steps into alkali-catalyzed transesterifications for laboratory or scaled-up process chemistry. In conversations with ink chemists, this flexibility stands out, as small tweaks yield tailored solvent packages for specialty applications—like heatset printing or low-odor formulations for sensitive locations. The chemical holds up in oxidative and reductive environments, so it doesn’t break down or polymerize unexpectedly during use. Reaction byproducts seldom build up to problematic levels if process parameters stay tight and materials meet spec. This sturdy backbone attracted attention well before sustainable chemistry became a driving theme in product development circles.
Walk into any supply warehouse and you’ll hear this chemical called by several names: 1-Methoxy-2-propanol, Dowanol PM, Propylene glycol monomethyl ether (PGME), and Propasol P. European papers sometimes list it as Propylene Glycol methyl ether, further broadening the alias set. Catalogs and MSDS sheets flag the same CAS number 107-98-2, offering a safety net in procurement when cross-checking among global sources. These aliases can trip up anyone less familiar with the territory, but learning to spot these names makes life easier for purchasers and lab staff alike. Standardizing purchasing and stockroom language helps avoid order mismatches and accidental substitutions, which, from experience, can set a whole production lot back by days if a less suitable glycol ether sneaks in.
Ventilation and PPE aren’t optional with 2-Methoxy-1-propanol. Exposures above regulatory thresholds lead to headaches and throat irritation, and frequent handling causes skin dryness. The fire risk at summer warehouse temperatures forces prudent manufacturers to segregate stockpiles away from heaters and open flames. Material Safety Data Sheets advise limiting time in poorly ventilated areas and using gloves and goggles for lineside workers. Occupational exposure guidelines—like those from OSHA and the European Union—set time-weighted averages at 100 ppm and flag low acute toxicity but highlight possible longer-term concerns with chronic exposure. Spill protocols prioritize quick containment and cleanup with absorbents and fume extraction. The push for automation and enclosed feed systems over open pouring reflects tough lessons learned from incidents and safety drills. Investing in local extraction, vapor alarms, and clear signage around transfer points saves both health claims and lost batches.
Paint and coatings get the headlines, but the real excitement comes from the edges—electronics cleaning, inkjet printing, plasticizer production, latex compounding. 2-Methoxy-1-propanol offers painters the benefit of quick drying with reduced brush drag. In ink rooms, it dissolves stubborn pigments and resins without gumming up rollers or plates. Cleanroom maintenance teams find it useful for removing residue from optical assemblies. Pharmaceutical plants use it as an intermediate or reaction medium when low toxicity and water-miscibility are important. Across sectors like adhesives and sealants, this glycol ether gives formulators an option that splits the difference between speed and solvency power. Its ability to bridge water and oil phases lets chemical engineers develop hybrid products that otherwise would demand two or more older solvents.
Academic and industrial labs keep probing for new uses and derivatives, drawn by the broad compatibility of 2-Methoxy-1-propanol. Recent years have seen work on greener synthesis methods, including bio-based feedstocks from glycerol and sugar alcohols. The push to cut emissions and energy use keeps teams experimenting with lower temperature routes and catalysts that minimize byproducts. At the bench, chemists run reaction screens to attach new groups, or to tweak evaporation dynamics for custom applications in printing electronics and specialty polymers. Advances in analytical instrumentation—especially NMR and chromatography—let manufacturers catch even slight shifts in composition, which plays into tighter batch control. The search for safer, more sustainable solvents focuses research on toxicity, persistence, and recyclability. Many standard university projects look at structure-activity relationships, seeking more selective or less persistent alternatives while building on this glycol ether’s backbone.
Compared to older aromatic solvents, 2-Methoxy-1-propanol doesn’t trigger as much concern, but ongoing animal studies illuminate possible longer-term effects on liver and reproductive health. Its acute toxicity sits at moderate to low levels; LD50 data across mammals show most hazards arise from repeated or high-concentration contact. Early studies flagged minor kidney effects in rodents, but follow-ups highlight that exposures in industrial settings rarely match these laboratory conditions. Regulatory agencies keep reviewing workplace limits based on emerging data, watching metabolites and breakdown products for unexpected bioaccumulation. Field experience lines up with current guidelines: proper engineering controls prevent issues for the majority of workers. Lower volatility also reduces incidental exposure for end users compared with ancestral solvents. Nonetheless, safety offices require education and regular reminders that moderate hazard doesn’t mean no hazard. Toxicological research continues at national institutes and universities, as the next generation of glycol ethers gets benchmarked for improved safety profiles.
Growing regulatory scrutiny of solvents pushes the industry to embrace glycol ethers like 2-Methoxy-1-propanol, especially as European REACH directives tighten allowable thresholds for VOCs and worker exposure. Consumer goods manufacturers want more environmentally responsible solvents without giving up the processing benefits they’ve come to expect. This chemistry finds its way into waterborne industrial coatings and inks built for rapid drying and low emissions. The trend toward water-based systems—driven by construction, furniture finishing, and automotive repair—means demand should hold steady, or even climb, in the years ahead. Advances in sustainable feedstocks bring talk of renewable glycol ethers, and research into hybrid formulations only expands the chemical’s reach. For production managers and chemists alike, 2-Methoxy-1-propanol brings fewer headaches and more flexibility, something that will likely keep this compound on procurement lists well into the future.
2-Methoxy-1-Propanol isn’t a household name, but it quietly plays a key role in industries many folks rely on. Most people bump into it through jobs in manufacturing, painting, or printing, even though they may never know its proper label. Many call it propylene glycol methyl ether for short, but in a paint shop or a print room, it’s just another part of getting work done.
Walk through any hardware store and take in the rows of paint cans. One thing holding that paint together, keeping it smooth, ready to coat walls or wood, is a solvent like 2-Methoxy-1-Propanol. This chemical helps thin out paint so it glides on with fewer streaks and clumps. There’s a similar logic in printing. Anyone who’s printed banners or read glossy magazines has probably benefited from this solvent. Ink must stay wet enough to move smoothly through machines, but dry fast on paper. 2-Methoxy-1-Propanol hits that balance, keeping shop floors running and magazine pages sharp.
Factories count on chemicals to cut through grease and grime, especially after a long shift. Here, 2-Methoxy-1-Propanol fuels spray bottles and wipes. It can dissolve sticky residues, helping machines run better and last longer. In automotive shops, techs find it helpful for cleaning parts, getting rid of build-ups before engines go back together. At home, it’s more rare, but some specialty cleaners might include it for the punch it packs.
Smartphones, tablets, and TV screens all need delicate cleaning during production. Large factories choose solvents that can clean glass or circuit boards without leaving streaks or scratches. This chemical ticks that box. Electronics are demanding—one stray fiber or oily smudge can cause trouble in a small device—so the right cleaning agent matters. Years ago, in a stint at a factory, I watched workers wipe each screen with swift, careful hands. The cleaning solution helped make sure nothing ruined the final product. There aren’t many options that get it done as safely and efficiently in one step as 2-Methoxy-1-Propanol.
2-Methoxy-1-Propanol’s power carries risk. People working with it breathe in fumes or touch it while cleaning or painting. Symptoms might start simple—headaches or dizziness—but over time, bigger concerns follow, like damage to liver, kidneys, or even reproduction. The European Chemicals Agency and others have called for tougher rules around its use. In my own time cleaning screens, I saw coworkers shrug off gloves or skip ventilation, not realizing long-term habits matter more than one-off exposures. People—the folks on the front lines—need better gear, information, and safer alternatives if possible.
Companies don’t always have easy answers. Chemical solvents aren’t always easy to swap out. Still, switching to water-based paints and cleaners, improving ventilation, and enforcing glove and mask use can cut trouble before it starts. Training sessions led by people who’ve spent days on the factory floor also help. Practical advice—how to open a barrel safely, or which mask fits a bearded face—makes a bigger difference than posters about chemical safety. When shopping for products or picking contractors, I always ask about their safety habits. From experience, little choices add up, often more than a shiny new chemical formula ever will.
2-Methoxy-1-Propanol can easily sneak by under the radar, since it shows up as a clear, faintly sweet-smelling liquid. In labs and on factory floors, it goes into cleaners, paints, inks, and coatings that you probably use every week without a second thought. Still, its friendly look doesn’t mean it’s harmless. The fumes and liquid irritate skin, eyes, and airways, and in bigger doses, there’s a real risk to internal organs like the liver and kidneys. I’ve seen people ignore the warnings—rushing through jobs, wiping sweat off their brows with gloves that had chemical traces. That always ends with someone learning the hard way.
Goggles and gloves never feel stylish or convenient, but skipping them leaves you playing with fire. 2-Methoxy-1-Propanol seeps through regular latex gloves, so nitrile or neoprene stand as safer bets. Splash-proof goggles shut out the stinging fumes and accidental splashes that seem to come out of nowhere. A small droplet on skin can cause burning or a rash, and in tight workspaces I’ve watched even careful people get caught off-guard by an unexpected spill.
In my early years, dusty rooms and chemical vapors gave me headaches and watery eyes, until an old hand marched me out to the loading dock and said, “Fresh air fixes a lot.” With 2-Methoxy-1-Propanol, decent ventilation does more than make you comfortable—it cuts how much you inhale. Open windows, exhaust fans or a proper fume hood make a huge difference. Lab workers know that sniff test you sometimes do by accident leads to throat irritation and dizziness. A good respirator (rated for organic vapors) stands at the ready in situations where air can’t keep up.
I’ve watched chemical splashes eat holes in jeans and stain shirts. Lab coats, aprons, and long sleeves act as frontline defenses. At the end of a shift, washing hands (and sometimes arms) matters as much as locking up the chemical cabinet. Spilled solvent left on surfaces or in open containers lingers and puts off fumes all day, so cleaning up spills and sealing containers tightly should be routine, second nature.
Every workplace keeps some forgotten shelf full of cans and bottles. That forgetfulness causes trouble. 2-Methoxy-1-Propanol belongs in a cool, well-ventilated place, away from open flames, sparks, or other chemicals that react. Containers with leaking lids or worn labels spark confusion and accidents. Good labeling, regular checks, and a no-nonsense rule about reporting damage keep those accidents rare.
Nobody feels proud fumbling with an eyewash station. Still, having water, a shower, and clear escape routes ready means nobody panics. I always tell new folks—know where to run if something goes wrong, and rehearse it in your mind until it feels automatic. Reporting exposure early beats stubborn silence every time; I’ve witnessed coworkers shrug off a splash until it grew into a blister or burn.
For all the safety gear and warning signs, familiar routines shape real protection. Work with the stuff in small amounts, bring your focus up a notch, and don’t let shortcuts lure you in. Solid habits, reinforced daily, make all the difference between a scare and a safe shift. That sense of caution isn’t about fear, but a respect for what chemicals can do if your guard drops. With 2-Methoxy-1-Propanol, I trust habits and clear thinking more than any single glove or gadget.
2-Methoxy-1-Propanol, also called propylene glycol methyl ether, pops up in plenty of shops stocking paints, coatings, or cleaning products. I’ve watched this liquid work in a paint shop: Clear as water, with a faint smell that hangs somewhere between sweet and chemical. Its texture is a lot like rubbing alcohol, and it doesn’t take much for it to slip between your fingers if spilled. On a basic level, it shows up as a colorless liquid.
It boils at about 120°C (248°F), but you won’t notice any drama as the temperature creeps up—it just quietly evaporates. It gets along nicely with water and most of the solvents a paint technician might keep under the bench, so it blends smoothly into water-based or solvent-based mixes. It stands up well in a bottle, with a freezing point down around -96°C, so cold storage really isn’t a worry. Its vapor slips into the air faster than glycerin’s, but not as fast as straight acetone, which helps open and adjust formulas in coatings.
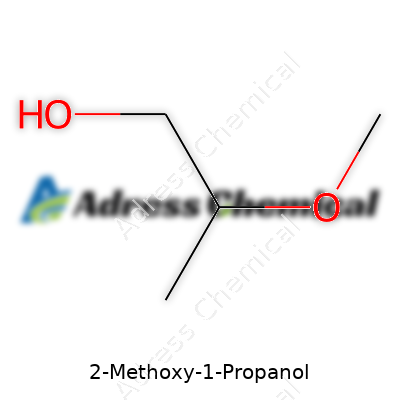
On the chemical side, the molecule champions flexibility. The ether bond (oxygen with two carbon friends) and the alcohol group (good old –OH) both go to work. The alcohol group brings in hydrogen bonds, which means it not only blends easily but also helps grab onto water-loving surfaces. The ether portion opens up the molecule to dissolve greasy, oily, resin-rich bits, which makes it a favored tool for folks in resin and ink manufacturing.
Take a glance at the numbers: Its chemical formula is C4H10O2, with a molecular weight of just over 90. Liquids of this kind don’t go up in flames like gasoline, but ignoring fire risk would be a mistake—it catches at about 44°C (111°F). Volatile but not reckless: that’s how I’d describe its flammability. It doesn’t turn sour or unstable if you leave it out, but it still deserves some respect. Solvent users know, one spark and proper ventilation suddenly matters a lot.
In printing shops or paint production, 2-Methoxy-1-Propanol makes life smoother. It acts as a coalescing agent, helping all the paint’s ingredients come together for a streak-free finish. Folks who deal with stubborn stains know this is a go-to in cleaning formulations, especially for grease and dried ink. I’ve seen janitorial supply buyers pick this solvent because it doesn’t fight with most surfaces—wood, tile, even metals—while rinsing away without fuss.
Paint makers love it for its low odor and mild skin irritation, especially compared to older, harsher solvents. If you’re mixing something in a small shop or a big plant, everyone wants formulas that don’t choke the air with fumes or leave behind residue that wrecks the final coat.
This solvent has been reliable for decades. Still, there’s an elephant in the room—solvent safety. Breathing too much, spilling on skin, waiting for headaches: these risks add up in day-to-day work. Regulations push for safer working environments, so newer blends with even lower toxicity are gaining ground. Replacing it takes more than swapping out one bottle for another, though. Every new chemical on the floor needs training, new ventilation planning, sometimes even a look at local water treatment, since water solubility means runoff into drains.
In the meantime, safe storage, gloves, goggles, and steady training keep folks safe. The world of paint and cleaning looks for “greener” answers, but until then, 2-Methoxy-1-Propanol handles a tough job with fewer trade-offs than most. Controlled use, practical safety steps, and ongoing research keep it a staple in the years to come.
Working in industrial settings, I’ve seen chemicals like 2-Methoxy-1-Propanol get handled daily. On the surface, it’s just another solvent in a long list, tucked into paints, inks, cleaning agents, and electronics manufacturing. Its role seems simple: to dissolve and mix. But the comfort of routine sometimes hides deeper risks that deserve attention.
Breathing in 2-Methoxy-1-Propanol doesn’t seem dramatic at first. Maybe a hint of sweetness in the air, maybe a quick fluid splash when cleaning a printhead. Over time, though, people who work with it regularly tell another story. The main worry comes from its ability to get through the skin and enter the bloodstream. Workers have experienced nausea, headaches, and irritation of the eyes and throat. Some research points to effects on the liver and kidneys after heavy exposure, though evidence in humans still grows.
Regulatory agencies haven’t set rock-solid exposure limits everywhere, but places like the European Union and the US generally see enough hazard to classify it as a substance that can cause serious eye irritation and might harm unborn children. In other words, workers who are pregnant or planning to become pregnant face real risk. The chemical’s relatives, like ethylene glycol ethers, have left a legacy of fertility problems and birth defects in animal studies. This isn’t a story built on panic—it’s built on lab data and a handful of hospital admissions.
What escapes down a factory drain doesn’t just vanish. 2-Methoxy-1-Propanol breaks down in water and soil, but its first stop in the ecosystem matters. Fish and aquatic insects can’t filter it out as well as people hope. Even short-term, high concentrations threaten aquatic life. That’s part of the reason some countries have asked industries to test wastewater for traces.
Eyes widen once you start thinking bigger. Spills in storage yards and leaks in transport create a chain of contamination from the ground into the water supplies. A handful of solvent can change the local chemistry, seeping into soil, hitching a ride on rainwater. Because people can’t see it or smell it once diluted, damage often skips beneath the radar until a problem turns up in fish kills or odd water taste months later.
In my time walking factory floors, the best defenses always come from practical steps. Ventilation makes a difference, but personal protective equipment like gloves and goggles builds a stronger barrier. Substitute less hazardous solvents where possible—a job that means both research and habit changes, not just wishful thinking. Training people to respect these chemicals, not to fear them, leads to better routines: washing hands before eating, labeling containers, never assuming a clear liquid is harmless.
On the environmental side, enforcing spill controls and requiring companies to treat wastewater before release shows respect for the community outside the fence. Environmental managers need real authority to stop shortcuts. Community watchdogs, who ask about chemical storage and wastewater plans, tip the balance from secrecy to shared safety.
Chemicals like 2-Methoxy-1-Propanol don’t make headlines often, but quiet risks add up over years. Knowing what sits inside that drum or is smeared on a workbench means better choices for both workers and their neighbors. Awareness drives change, and in the case of this solvent, the stakes go far beyond a day’s output or a single batch of paint.
Folks working in labs or factories sometimes treat 2-Methoxy-1-Propanol like just another clear liquid. A simple label and back on the shelf — out of sight, out of mind. I’ve seen more than one workplace shrug off the careful handling steps, hoping to cut corners and save time. That attitude quietly sets the stage for trouble, because this chemical comes with its own set of risks no one should handwave away.
Ask anyone who’s spent weeks organizing a chemical storeroom: solvents build up fast, and sometimes folks try storing them like soup or paint. That spells danger. 2-Methoxy-1-Propanol, for example, needs a cool, dry place, away from direct sunlight or heat sources. Why? High temps push up evaporation rates and spoil the stability of the chemical. Trying to stack drums near a heat vent or in a corner without air circulation means rolling the dice with leaks or dangerous vapors.
I once watched someone pop a battered plastic jug of this stuff on a top shelf, assuming it was fine. Over time, pressure built up and the lid warped. Good thing we caught it before it started leaking. Using steel or sturdy plastic containers with tight-fitting lids helps avoid that headache. Not every shelf or cabinet works either. Metal shelving rusts and corrodes if spills go unnoticed. Chemical storage cabinets specifically rated for flammable liquids — usually with proper ventilation and spark-proof fixtures — cut the risk of nasty accidents down to nearly zero.
Labeling, though basic, can’t get skipped. Faded or missing labels invite mix-ups. In busy spaces, it’s not hard for someone to grab the wrong solvent for a procedure, especially if they’re new. Legible, waterproof labels with the full name and hazard info keep everyone in the loop.
Loading a drum of 2-Methoxy-1-Propanol into a pickup bed and hitting the road isn’t the same as lugging sacks of fertilizer. Flammable liquids hit the Department of Transportation’s regulations fast. They want containers that hold up to bumps and stops, and they aren’t kidding about spill containment. Closed, upright drums in secure racks make a big difference.
Even as a driver, I’ve felt uneasy hearing a drum thud in the back at a red light. Unrestrained containers move, sometimes rolling enough to mark the end of a long trip with a lab bill or a call to hazmat. Most carriers now use cages or custom-designed frames that hold these drums steady. No one wants a puddle forming at the bottom of a delivery truck.
If you’ve ever transferred solvents from one location to another, you know the paperwork weighs nearly as much as the chemical. Shipping papers, emergency contacts, hazard class numbers — they all matter. Law enforcement or cleanup crews don’t appreciate being left in the dark during an accident.
A lot of us learn by seeing, and sometimes the consequences don’t sink in until someone gets sick or equipment gets damaged. 2-Methoxy-1-Propanol exposure can cause headaches, nausea, or worse if safety instructions get ignored. Having a spill kit, fresh gloves, and goggles handy isn’t just for show. It’s looking out for your coworkers, yourself, and anyone who steps into the storeroom or truck.
This isn’t about paranoia — it’s about solid practice. Training sessions every so often remind new folks and old hands what matters, and reviewing incident reports helps everyone learn, not just the people involved. In my own experience, that’s what turns a workplace into a safer, smarter place, instead of just ticking off a compliance box.
| Names | |
| Preferred IUPAC name | 2-Methoxypropan-1-ol |
| Other names |
Propylene glycol monomethyl ether 1-Methoxy-2-propanol PGME Dowanol PM Propylene glycol methyl ether |
| Pronunciation | /tuːˈmɛθ.ɒk.si.wʌnˈprəʊ.pə.nɒl/ |
| Identifiers | |
| CAS Number | 1589-47-5 |
| Beilstein Reference | 1209230 |
| ChEBI | CHEBI:8178 |
| ChEMBL | CHEMBL155597 |
| ChemSpider | 7309 |
| DrugBank | DB02307 |
| ECHA InfoCard | ECHA InfoCard: 02-2119752475-32-0000 |
| EC Number | 01-604-029-00-7 |
| Gmelin Reference | 81147 |
| KEGG | C02310 |
| MeSH | D020064 |
| PubChem CID | 7922 |
| RTECS number | UB7700000 |
| UNII | 7XJF9DPQ5D |
| UN number | UN3092 |
| CompTox Dashboard (EPA) | DTXSID0023151 |
| Properties | |
| Chemical formula | C4H10O2 |
| Molar mass | 90.12 g/mol |
| Appearance | Colorless liquid |
| Odor | weak ether-like |
| Density | 0.924 g/mL at 25 °C |
| Solubility in water | miscible |
| log P | 0.437 |
| Vapor pressure | 0.76 mmHg (20°C) |
| Acidity (pKa) | 15.1 |
| Basicity (pKb) | pKb = 5.62 |
| Magnetic susceptibility (χ) | -51.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.404 |
| Viscosity | 2.3 mPa·s at 20 °C |
| Dipole moment | 2.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 214.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -448.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2384 kJ/mol |
| Pharmacology | |
| ATC code | D01AE22 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H336 |
| Precautionary statements | P210, P264, P280, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 75 °C (closed cup) |
| Autoignition temperature | 287°C |
| Explosive limits | 1.5–13% |
| Lethal dose or concentration | LD50 oral rat 5660 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 5660 mg/kg |
| NIOSH | SN: 8054 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 25 ppm |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Propylene glycol 1-Methoxy-2-propanol Dipropylene glycol methyl ether Methoxyethanol Ethylene glycol |