History often shapes the path of a chemical’s reputation and its place in the market. Back in the early twentieth century, as the chemical industry explored new ways to blend solvents into emerging paint and cleaning products, 2-ethoxyethanol stepped into the spotlight. Chemists noticed its knack for dissolving stubborn resins and its ability to mix with both water and oil-based components. The boom in consumer products during the mid-1900s drove demand, as factories cranked out everything from lacquers to antifreeze for cars parked on frozen streets. Regulations came later, and only then did the chemical’s downsides take center stage. Earlier, its potential toxic effects did not cause much worry. Once studies linked exposure to health issues, voices grew louder for more controls, especially for people facing regular workplace contact. The journey of 2-ethoxyethanol mirrors shifts in how society balances innovation and safety.
2-Ethoxyethanol counts as a glycol ether, a group often favored for their ability to dissolve greases, oils, and glues where water falls short. Big players in the coatings industry, ink production, and pharmaceuticals look for this solvent when they need something with both strength and finesse. Businesses value it because it softens and penetrates substances that resist other liquids, making it a workhorse for surface cleaning preparations, textile processing, and degreasing agents. Its role in production lines translates into smoother operations, fewer stoppages, and less time wasted wrestling with stubborn residues.
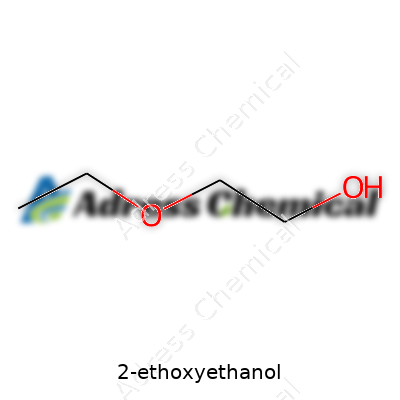
This liquid brings a mild, almost sweet odor and stays clear without clouding up over time. It flows easily and absorbs water from the air, showing its hygroscopic side. Boiling point sits just above 135°C, high enough to stick around in finishing applications but not stubborn enough to slow drying. At the molecular level, the chemical blends an ethoxy group with a two-carbon backbone carrying a hydroxyl group—a structure that gives both solvent power and water solubility. Flammability draws needed concern in busy warehouses and labs. Its vapor can light up under the right conditions, so storage and handling happen far from sources of sparks. In bulk, it can strip paint and dissolve polymer coatings, hinting at strength while still cleaning up with water.
Labels and safety data sheets for 2-ethoxyethanol warn about inhalation and skin contact, following both industry standards and legal rules. The product must arrive with clear purity grades, usually above 99%, and minimum water content because water affects its performance. Companies expect certificates of analysis proving low traces of aldehydes, chlorides, and heavy metals—these contaminants can disrupt polymerization and other reactions. Transport containers get distinct hazard symbols, marking both toxicity and flammability. Workers rely on up-to-date labeling to follow safe handling and storage, knowing that clear communication keeps accidents down and compliance up.
Making 2-ethoxyethanol takes a straightforward route using ethylene oxide and ethanol under controlled pressure and temperature. The process, often called ethoxylation, works by letting ethanol react with ethylene oxide, sometimes with a catalyst to speed up the reaction and boost yield. Air-tight reactors cut down byproduct formation and help ensure the final solvent measures up to the specifications buyers need. Post-reaction, distillation separates the pure chemical from contaminants, with waste streams carefully managed to avoid environmental harm. Plant operators watch for leaks and pressure swings, as these steps require both skill and close attention.
2-Ethoxyethanol tends to get involved in esterification, etherification, and sometimes oxidation reactions. It dissolves not only resins and pigments but also plays a part in producing other chemicals via nucleophilic substitution. Chemists might transform it into 2-ethoxyethyl acetate, another valued solvent, using acetic acid in a mild acid-catalyzed process. Oxidative pathways may lead to aldehydes or acids, each with their own use in surfactant or plasticizer production. Reactivity often depends on the hydroxyl group, so modifications usually target that end of the molecule.
Walk through supply catalogs or scroll through chemical indexes, and you’ll see this substance under names like ethylene glycol monoethyl ether, EGEE, Cellosolve, and ethyl cellosolve. Some industries swear by trade names, making supply management harder without careful checks. Globally, the same compound may arrive with a varied label depending on the producer or region, so tracking synonyms keeps both buyers and lab techs out of confusion. Most regulatory documents mention the CAS number (110-80-5) for certainty across language and border gaps.
2-Ethoxyethanol has earned a reputation for risk that shapes every part of its handling. Regulations from the Occupational Safety and Health Administration (OSHA) and the European Chemicals Agency (ECHA) limit airborne exposure and demand strict controls for people working with the liquid or its vapors. Proper use of gloves, goggles, splash aprons, and ventilated workspaces becomes part of daily routines in labs and paint shops. Regular health checks—for blood counts, kidney, and liver markers—help catch early signs of trouble. Emergency showers, eye washes, and spill kits sit close by in factories where drums of this solvent arrive. Air monitoring keeps invisible vapors from creeping above recommended limits. Environmental rules control how leftover material gets neutralized or disposed, treating every drop as something not to let slip into the water table unchecked.
The draw of 2-ethoxyethanol runs strong in the coatings and printing industries, where it boosts flow and level of inks on presses and rollers. Painters favor it in systems that demand smooth spread and careful drying between layers. Plastic and polymer manufacturers turn to this solvent when blending complex mixtures where lesser chemicals fail. Electronics assembly uses it as a cleaner for delicate parts, while textile dyers rely on it for its wetting and penetration powers. In pharmaceutical manufacturing, it finds a niche for extracting plant compounds and preparing certain medicines, though stricter purity and safety rules apply due to health concerns. Given tightening global regulations, replacements often get considered, but technical demands mean high-volume users keep it in rotation for jobs not easily switched to green alternatives.
Scientists continue looking for ways to cut the risks and meet growing calls for greener chemistry. Research teams now explore modified glycol ethers with reduced toxicity, scanning for molecules that keep cleaning power but lose the health hazards. Analytical chemists refine ways to spot tiny traces in manufacturing environments, helping keep exposures beneath legal thresholds. Universities and R&D centers run studies comparing workplace exposure outcomes and test alternative solvents for the same jobs—revealing trade-offs in efficiency, price, and safety. Some projects try blending this chemical with others to lower required amounts, balancing performance against limits set by regulators and corporate health teams. Green chemistry comes up often at industry seminars, pushing newer solvents sourced from renewable feedstocks, but for now, 2-ethoxyethanol holds onto key spots thanks to legacy performance.
Decades of animal and human studies build a strong case for caution. Scientists report effects on the central nervous system, bone marrow, and the reproductive system after repeated or prolonged exposure, especially through skin and inhaled vapor. Organizations such as the National Institute for Occupational Safety and Health (NIOSH) classify it as hazardous, setting exposure limits well below the point of acute symptom onset. Medical surveillance in workplaces links higher rates of anemia, fertility challenges, and occasional cases of kidney and liver trouble among exposed groups. Animal tests point to developmental risks, including fetal toxicity, leading both the European Union and U.S. authorities to flag high-use occupations for increased oversight. Toxicologists recommend barrier creams, swift spill responses, and pollution controls on plant exhaust streams.
Growing environmental awareness and worker safety activism shape the future of 2-ethoxyethanol. Companies face mounting pressure to adopt alternatives, especially in paints, cleaning solvents, and pharmaceuticals where health impacts can add up over time. Research investments flow toward bio-based solvents and advanced glycol ethers with tailored profiles to reduce both toxicity and environmental load. Regulatory tides keep pushing exposure limits lower, leading downstream users to design products that either use less or swap out this solvent altogether. The next decade looks like a tightrope walk between delivering on performance and meeting social responsibility standards. Some countries have started phasing out broad uses, while research into safe disposal, recycling, and substitution keeps growing. As end-user awareness widens, labels and technical data sheets call for clearer hazard communication. Demand for more sustainable choices keeps pushing both established producers and new entrants to rethink what comes next in industrial solvents.
Walk into an auto repair shop and you pick up the unmistakable hint of solvents in the air. Factories, paint shops, and even some household cleaning product producers count on these chemical helpers. One familiar compound in this invisible world is 2-ethoxyethanol, often called ethyl cellosolve. It’s a colorless liquid with a pleasant odor, but the way it does its job matters far more than how it smells.
No single substance carries paint on a brush or ink across a page by itself. Paints, varnishes, stains, and printing inks must stay smooth, workable, and easy to spread—not too thick, not too runny. That’s where 2-ethoxyethanol steps in. Acting as a solvent, it keeps pigments and resins evenly mixed. In my years working with industrial-grade paints, products using 2-ethoxyethanol went on smoother and dried with fewer streaks, especially on tough surfaces like metal or glass.
Another area where this chemical shows up is in cleaning products meant for tough jobs. Some grease and oil stains just laugh at water and soap. Add 2-ethoxyethanol, and you get a cleaner that breaks up grime with far less scrubbing. This advantage matters in places like print shops or factories where downtime costs money and a clean workspace is non-negotiable.
Beyond paints and cleaners, manufacturers use 2-ethoxyethanol for making lacquers, dyes, and even certain pharmaceuticals. Chemical engineers prize it for how well it interacts with both water and oil-based materials. One time on a consulting project for a small plastics company, we needed a solvent that wouldn’t react badly with their blends or leave behind a residue. Testing showed 2-ethoxyethanol let the pigments bond tightly to the plastic, broadening the range of colors and finishes they could offer.
Tough jobs demand strong tools, and 2-ethoxyethanol is no exception. Working around it, I learned you can’t ignore safety. Chronic exposure at high levels damages the blood, liver, and kidneys, according to research from the National Institute for Occupational Safety and Health. Short-term exposure causes headaches and skin irritation, and long-term contact raises reproductive risks. I’ve seen managers put better ventilation and personal protective equipment in place after learning how quickly vapors become a health concern, even when the substance seems harmless at first glance.
Looking for replacements isn’t always easy. There are other glycol ethers, but many bring their own hazards or don’t work as well. Some teams now use automation to cut down on direct human contact, pairing that with stricter air monitoring and robust training programs. The goal has become reducing how much makes it into the workplace air, not just handing workers thicker gloves and saying that’s good enough.
Government agencies continue to update safety regulations, pushing industries to find the safest approach without losing performance. The push for greener chemistry has also led to more research into less toxic alternatives, but old habits and product formulas change slowly.
Like many industrial solvents, 2-ethoxyethanol plays an essential but invisible role in products we use every day. Its benefits—strong cleaning power, smooth application, and flexibility—must always come balanced with a careful eye on health and safety. As technology moves, responsible choices and new options can lessen the risks and make workplaces better for everyone.
2-Ethoxyethanol shows up in unexpected places—paints, varnishes, inks, and cleaners. I remember seeing safety warnings in a print shop years back, with labels cautioning the staff to wear gloves whenever they handled the cleaning solvents. Few of us, outside scientific circles, probably stop to think about the actual stuff behind those warnings, but 2-ethoxyethanol is one reason those gloves matter.
Health experts have flagged this chemical for good reason. Short-term effects crop up fast—puffy eyes, sore throats, headaches, and nausea. Spend enough time around it without proper protection, and the dangers dig deeper. The U.S. Environmental Protection Agency states that extended exposure can impact the blood, bone marrow, and reproductive systems. Workers in factories and print shops have reported fatigue, anemia, and even fertility problems after repeated contact. These aren’t scare stories; they come from real workplace studies.
Most risks show up in workplaces, not in everyday home life. Factories that use industrial cleaners or paint products quickly become risky spots if ventilation doesn’t keep fumes in check. The smell gets strong, and eye stinging sets in if masks or goggles slip off. I’ve spoken to friends in auto body shops who talk about headaches and dry skin—problems they never linked to the solvents until someone pointed out the safety data sheets posted by the doors.
The health effects go beyond simple irritation. Scientists have noticed changes in blood cells, which can cause anemia. There’s data showing links to lower sperm counts and trouble with pregnancies in people exposed for long stretches. Studies in animals have raised red flags for possible birth defects, which points to why governments in the European Union and the United States demand specific warnings on labels. Long-term health issues don’t care much about whether someone ignored the problem; over time, effects stack up quietly.
You can’t always smell it. It sneaks through the skin or slides through a fabric that isn’t chemical resistant. Respirators, gloves, and ventilation systems turn into the only real shields. OSHA caps how much someone can breathe in while at work—set at 200 parts per million. These rules land in place because ordinary soap and water can’t always reverse the damage.
There’s nothing fancy about the best fixes here. Labels need plain talk and not just tiny print buried on the side. Training workers doesn’t just mean tossing a manual their way, but walking them through the real risks with practical demos. Employers can swap to safer products where possible—many water-based paints get the same job done. Companies owe workers the right kind of gloves, masks, and ventilation. Health checks that catch warning signs early can save a lot of pain down the road.
Getting careless about these chemicals gives health problems room to grow. Personal experience tells me that once someone starts paying closer attention—cracking a window open, pulling on gloves, demanding better ventilation—the everyday risk sharpens into focus. People should never have to trade safety just to finish a shift or get the job done faster.
Working with 2-ethoxyethanol brings some serious safety concerns. This solvent has a knack for slipping through gloves, drifting through the air, and finding its way into the body. I’ve spent time in labs and on shop floors where this chemical showed up, and it always got my respect. Breathing in vapors or letting it touch your skin isn’t just uncomfortable—it can stir up everything from headaches and nausea to much heavier health issues, including damage to blood and organs over long exposure. These risks aren’t theoretical, either. Centers for Disease Control (CDC) flag this compound for reproductive harm and other chronic problems.
Personal protective equipment matters, plain and simple. Shorts and t-shirts won’t cut it. Gloves that hold up to solvents—think nitrile, butyl rubber, or laminates—make all the difference. Regular latex doesn’t stand a chance against this stuff. Safety goggles and splash-resistant face shields guard your eyes and face. A lab coat or chemical-resistant apron adds extra protection. Respirators come into the picture if the job leaves vapors hanging in the air. A tight-fitting PPE routine becomes as familiar as grabbing your keys before work.
There’s no shortcut for proper airflow. Fume hoods, local exhaust systems, and open windows all push chemical vapors out, making the air safer. In drafty garages or workshops, adding fans that point fumes away keeps things under control. Simple tricks like storing containers tightly sealed and labeling them well also help stave off accidental exposure. The sweet, ether-like smell of 2-ethoxyethanol should act as a warning that something’s amiss, not just another day in the lab.
Nobody enjoys a spill, and 2-ethoxyethanol can splash quick. Pour slowly, avoid rushing, and always transfer liquids using the right pumps, never by mouth pipetting. Work over spill trays when moving larger amounts. If a splash hits skin or clothing, washing up with soap and water beats just wiping it off. Chemicals like this stay active and dangerous long after the task ends, so proper disposal through hazardous waste channels matters. Never dump leftovers down the drain—municipal waste workers shouldn’t have to wrestle with toxic solvents down the line.
Experience in the lab shows that people slip up when they don’t really understand what they’re dealing with. Safety briefings, consistent signage, and real-time supervision all help. I’ve seen shops skip training, and it leads to avoidable close calls. Everyone who works with volatile solvents—new hires or veterans—benefits from clear instructions and repeat reminders. If anyone starts looking queasy, feeling dizzy, or getting odd symptoms during or right after working with 2-ethoxyethanol, seeking medical help fast proves crucial.
Engineers and manufacturers scan for safer substitutes every year, but sometimes, old chemicals stick around because nothing else gets the job done quite the same way. Companies benefit from keeping an eye open for new options that lessen harm. For now, those who use 2-ethoxyethanol control the risk through smarts, gear, and planning. Sticking to these principles lowers chances of accidents, protects people, and lets work move ahead smoothly.
2-Ethoxyethanol shows up as a colorless liquid. A quick sniff will tell you its faint, somewhat sweet odor can be hard to ignore. At room temperature, it flows easily, and you don’t see any cloudiness. I’ve come across this solvent in a handful of labs, and there’s never been a time it looked or acted like anything other than a typical organic liquid.
We aren’t just droppping facts with boiling at around 135°C (275°F) and freezing at about -70°C (-94°F). These points have real impact on safety and storage. That high boiling point means you can heat it for certain reactions without losing the whole lot to evaporation. Cold winters won’t turn this liquid to ice easily, saving a lot of trouble for workers in less controlled conditions.
2-Ethoxyethanol’s miscibility with water sets it apart. You pour it into water, and it blends completely. Same thing happens with solvents like ether, alcohol, or many hydrocarbons. This makes cleaning up after spills trickier, since water alone can’t just flush it away. I remember seeing teams scramble with absorbents and ventilation after a minor leak—water-washing only spread it thinner on the surface.
It gives off vapor easily enough at normal conditions—vapor pressure circles about 6 mmHg at room temperature. It won’t have people coughing up a storm in a matter of minutes, but closed rooms can build up fumes if you slack on ventilation. I never forget the lesson learned from a congested workspace—nobody wants a headache mid-shift just from breathing in too much solvent vapor.
Flash point clocking in near 43°C (109°F) signals it can go up in flames with enough heat and an ignition source. Since sparks aren’t rare around electric motors or friction, storing this solvent near open flame or in unventilated spaces sets the stage for danger. From first-year lab lessons to warehouse signage, clear warnings always accompany its use.
Density tells a simple story—at a touch over 0.93 grams per cubic centimeter, this compound is lighter than water. It floats on top if poured gently into a beaker of water. In situations involving accidental spills, this makes it more prone to spread than sink. Thin layers across surfaces complicate cleaning efforts and ventilation plans.
Every property above ties back into workplace safety and public health. That easy mixing means contaminated water can turn up downstream unless people track disposal closely. Lightness and volatility make for quick airborne exposure in tight labs. You won’t see 2-ethoxyethanol evaporate as fast as some smaller ethers or alcohols, but ignoring it still leads to chronic exposure problems. Long hours in its presence bring up headaches, and repeated skin contact can crack or dry skin fast. Gloves, goggles, and fans—these go from “nice to have” to “essential” every time this solvent gets pulled from a shelf.
Simple steps cut risks. Regular ventilation checks, real-time air quality monitors, and clear spill kits nearby change outcomes quickly. Substituting less volatile, less toxic solvents in non-critical processes limits accidental exposures. Early training sticks with workers, and ongoing updates ensure old habits don’t return as new risks sneak in. Looking at the big picture, up-to-date labeling and open reporting reduce not just accidents, but lingering health issues for anyone relying on this common solvent.
Storing 2-ethoxyethanol brings with it a level of responsibility that shouldn’t get swept aside for convenience. This solvent shows up across labs and manufacturing because it dissolves a wide range of chemicals. Yet, that same versatility means it behaves aggressively with common plastics and open air. I’ve seen overlooked storage practices put workers at risk, often because the liquid looks harmless. Any space used for 2-ethoxyethanol demands proper ventilation. Vapors don’t politely stay put. Without enough airflow or the right seals, you face a higher risk of exposure, and inhaling the vapor brings real health risks: headaches, fatigue, even impacts to your blood and organs if exposure keeps happening.
Choosing containers matters just as much as where they’re placed. Steel drums with tight-fitting lids work—storing the solvent in glass sometimes works, but only in small scale lab contexts where external breakage won’t spark a bigger disaster. Ordinary polyethylene jugs erode after a while, and leaks in storage aren’t forgiving, especially indoors. Often, people ignore the chemical compatibility charts. I tell anyone handling chemicals: check the manufacturer’s guidelines, not just once, but every season, because product specifications change and so does weather. Fluctuating temperatures ramp up pressure within sealed containers, and even small changes can cause a can to swell or leak.
Disposal isn’t as simple as dumping leftover solvent down a drain or mixing it with household trash. These shortcuts endanger water systems and risk heavy fines. I remember a local painter pouring a similar glycol ether into a storm drain after a job, only to suffer health problems later, along with a hefty penalty. Regulations call for hazardous waste pick-up, and most cities supply clear instructions—or names of companies that handle chemicals. Collect the used 2-ethoxyethanol in a compatible, clearly labeled container separate from normal garbage. Keeping it in an unmarked bottle creates risk for everyone—janitorial staff, waste handlers, even children who might enter a workshop.
Workers should not rely on gloves for magic protection. Ordinary latex gloves degrade. Nitrile gloves last longer but still need swapping if exposed for more than a few minutes. Spills soak into skin fast, and the health effects linger for months, sometimes longer. Anyone overseeing chemical disposal has to give specific training on handling, not just hand people a binder and trust the paperwork alone.
Some say all these rules slow things down. In reality, they help avoid problems that snowball. Fire risk hangs over all glycol ethers. Store the solvent far from ignition sources—easy advice, often skipped out of laziness. Fireproof cabinets and chemical storage lockers, with regular inspections, drastically improve safety. In shared spaces, everyone keeps track of expiration dates and partial containers. Unlabeled jugs end up forgotten, leaking, or misused during a busy day.
Adopting better storage and disposal faces the same old hurdles: cost, inconvenience, and habit. Institutions that prioritize safety—schools, hospitals, responsible manufacturing—build these steps into culture. Generating less waste helps too. Order what’s needed for the job and no more. Tap into community hazardous waste drop-offs if large quantities aren’t used regularly. Education beats emergency response every time.
2-ethoxyethanol won’t bite unless mishandled—but shortcuts catch up. With some forethought and respect for both the chemical and those exposed to it, accidents shrink and communities stay healthier.
| Names | |
| Preferred IUPAC name | 2-ethoxyethan-1-ol |
| Other names |
Ethylene glycol monoethyl ether Cellosolve Ethyl cellosolve Glycol monoethyl ether EGEE 2-Ethoxy-1-ethanol |
| Pronunciation | /tuː ɪˈθɒksiˌɛθənɒl/ |
| Identifiers | |
| CAS Number | 110-80-5 |
| Beilstein Reference | 1645228 |
| ChEBI | CHEBI:27819 |
| ChEMBL | CHEMBL1358 |
| ChemSpider | 7572 |
| DrugBank | DB02141 |
| ECHA InfoCard | 03a8017b-b49e-406d-993c-8d2c2a6e38bc |
| EC Number | 203-804-1 |
| Gmelin Reference | 8218 |
| KEGG | C06169 |
| MeSH | D004979 |
| PubChem CID | 8113 |
| RTECS number | KK8475000 |
| UNII | 2Z8I3LO3SU |
| UN number | UN1171 |
| CompTox Dashboard (EPA) | urn:epa.comptox.dashboard:DTXSID8023729 |
| Properties | |
| Chemical formula | C4H10O2 |
| Molar mass | 90.12 g/mol |
| Appearance | Colorless liquid |
| Odor | Ether-like |
| Density | 0.930 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.32 |
| Vapor pressure | 0.83 mmHg (20 °C) |
| Acidity (pKa) | 15.1 |
| Basicity (pKb) | pKb ≈ 5.15 |
| Magnetic susceptibility (χ) | -7.83 × 10⁻⁶ |
| Refractive index (nD) | 1.404 |
| Viscosity | 1.7 mPa·s (20 °C) |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 249.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -429.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2334 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | D07AX01 |
| Hazards | |
| GHS labelling | GHS02, GHS06, GHS08 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H312, H315, H319, H332, H360 |
| Precautionary statements | P201, P202, P210, P260, P264, P280, P308+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 43°C |
| Autoignition temperature | 285°C |
| Explosive limits | 3.8–15% |
| Lethal dose or concentration | LD50 oral rat 3300 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 2,460 mg/kg |
| NIOSH | K1600 |
| PEL (Permissible) | 200 ppm (parts per million) |
| REL (Recommended) | 18 mg/m³ |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Ethylene glycol Diethylene glycol Ethylene glycol monoethyl ether acetate Propylene glycol 2-Methoxyethanol |